Letter to the Editor: FDA Citizen Petition Seeks Historical Reckoning to Require the Labeling of Gluten on All Food Packages in the United States
Co-Founders of Celiac Journey, and Authors of FDA Citizen Petition to Require the Labeling of Gluten on All Packaged Foods (Docket: FDA-2023-P-3942)
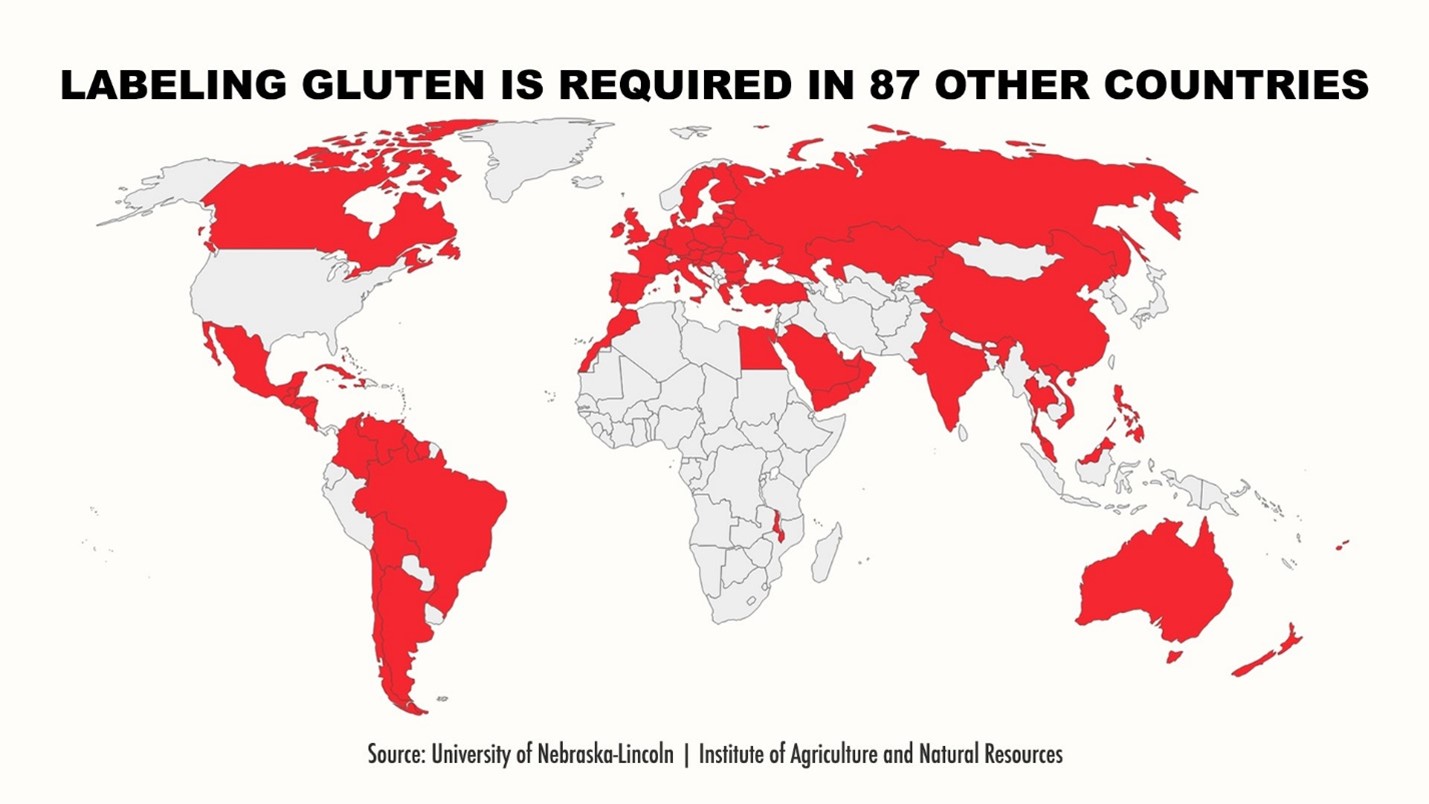
As a food allergy family, we are grateful to the Food and Drug Law Journal (FDLJ) for providing us with a road map of the history of the Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA).[1] The FDLJ has also helped us navigate our food policy journey and draft our FDA Citizen Petition, submitted on September 13, 2023, requesting that FDA require the labeling of gluten on all packaged foods in the United States just like gluten must be declared on all food labels in 87 other countries (FDA-2023-P-3942).[2]
In 2018, fate shined a bright light on the juxtaposition of food privilege and food insecurity through the lens of our family’s new Celiac community. After undergoing tests to determine why five-year-old Jax Bari was not growing, he was diagnosed with Celiac Disease, a potentially life-threatening and life-debilitating food allergy and auto-immune disease triggered by eating gluten, a protein found in wheat, barley, rye, and most oats.
We used to enjoy our family’s food privilege of being able to eat what we wanted, eat out where we wanted, all while savoring the spontaneity of food in life’s daily activities without constant anxiety about diet-related disease. Ingesting just a crumb of gluten can cause Celiacs to get violently ill for days and suffer adverse health effects including anemia, cancer, immunological scarring, intestinal damage, and malnutrition. Unlike other food allergies, one cannot outgrow Celiac, and there is no rescue medicine (i.e., epinephrine or antihistamine) to take for accidental ingestion. The only available treatment is a strict gluten-free diet for life.
Just before he started Kindergarten, Jax was anemic with severe intestinal damage. As he learned to read, Jax started with fairy tales and food labels, which are confusing and incomplete, often resulting in food insecurity caused by fear and uncertainty about the safety of a food product and accidental glutenings (ingestion of gluten).
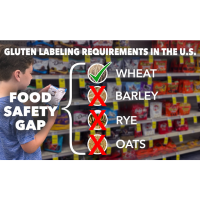
Since FALCPA became law on January 1, 2006, only wheat has been required to be labeled in the United States, but barley, rye, and oats have not.
Imagine if you had a tree nut allergy and only almonds were required to be labeled, but not other tree nuts such as pecans and walnuts. That would create a massive food safety gap that needed to be closed. Fortunately for those with a tree nut allergy, that scenario is not the case. But that similar safety gap does exist with the voluntary labeling of barley, rye, and oats for millions of American Celiacs like Jax.
According to Laura Derr’s 2006 article in the FDLJ, “When Food Is Poison: The History, Consequences, and Limitations of the Food Allergen Labeling and Consumer Protection Act of 2004”[3]:
“The clear labeling of wheat under the FALCPA, in fact, may have the perverse effect of harming those who must avoid gluten. A gluten-free product always is wheat-free, but the reverse is not true. Children or caregivers of children with Celiac Disease may assume incorrectly that a wheat-free product is gluten-free if they are not familiar with or do not remember the various terms for gluten-containing ingredients (e.g., rye, barley, and malt) besides wheat . . . . It is clear, however, that including gluten-containing grains besides wheat in the FALCPA’s allergen labeling scheme, expressly requiring FDA to consider Celiac Disease in its ingredient exemption decisions, and requiring the ‘gluten-free’ declaration on products without gluten could have gone significantly further to assist people living with this [Celiac] Disease.”[4]
One crumb is all it takes: gluten is kryptonite to our family’s little Superman. It has been heartbreaking when Jax has gotten glutened, and there is nothing that anyone can do to help him other than provide some minimal comfort and clean-up as his body starts attacking itself in an auto-immune cascade that can last for days.
there is nothing that anyone can do to help him other than provide some minimal comfort and clean-up as his body starts attacking itself in an auto-immune cascade that can last for days.
According to Beyond Celiac, one in five children with Celiac Disease is not healing on the gluten-free diet, and 44% of people who follow a strict gluten-free diet still get glutened (i.e., accidentally ingest gluten) once a month.[5]
Through our food policy journey, we came to rely on Derr’s FDLJ article.[6] According to Derr, FALCPA “does not preclude FDA from expanding via regulation the list of major allergens requiring identification under the FALCPA’s labeling scheme.”[7] Section 203(b) states that the labeling requirements established under new section 403(w) “do not prevent the Secretary from requiring labels or labeling changes for other food allergens that are not major food allergens.”[8]
Eating without fear is Jax’s goal! To that end, Jax’s Citizen Petition (FDA-2023-P-3942) is requesting that FDA better protect more than 3.3 million Americans with Celiac by labeling gluten (wheat, barley, rye, and oats) as a major food allergen on all packaged foods, just like labeling gluten is required to be labeled in 87 other countries.[9] We have requested that FDA issue a rule under its existing FALCPA authority (21 U.S.C. § 343(x)) to:
(1) require that all ingredients with gluten be listed by name in the ingredient lists of all foods and; and
(2) add gluten to FDA’s list of allergens in Sec. 555.250 of its Compliance Policy Guides Manual, “Statement of Policy for Labeling and Preventing Cross-contact of Common Food Allergens” to address both labeling and cross-contact issues related to food manufacturing practices.
If the labeling of gluten was mandatory on all products, our lived experience strongly suggests that the number of gluten-free products available to those who have Celiac would greatly expand. According to The New York Times, “because use of the gluten-free claim is voluntary, many foods that are in fact gluten-free might not be labeled as such.”[10] Voluntary labeling makes Celiacs beholden to a premium marketplace of food items in which the price of gluten-free foods is 2x–6x more on a per ounce basis than their gluten-containing counterparts.[11]
Labeling gluten is in alignment with the conclusions of international food safety authorities and expert committees comprised of scientists, regulators, physicians, clinicians, individuals, and risk managers from academia, government, and the food industry, including the 2021 Food and Agriculture Organization (FAO) of the United Nations/World Health Organization (WHO) Expert Consultation on Risk Assessment of Food Allergens, which included FDA’s Dr. Lauren Jackson, Chair, and FDA’s Dr. Stefano Luccioli (“2021 FAO–WHO Expert Consultation”). The 2021 FAO–WHO Expert Consultation found, “[b]ased on systematic and thorough assessments which used all three criteria (prevalence, severity and potency), the Committee recommended that the following should be listed as priority allergens: Cereals containing gluten (i.e., wheat and other Triticum species, rye and other Secale species, barley and other Hordeum species and their hybridized strains) . . . .”[12]
In addition, FDA has received supporting comments on Jax’s Citizen Petition from Children’s Hospital of Philadelphia, Harvard Medical School/Beth Israel Deaconess Medical Center, Consumer Reports, National Celiac Association, FARE (Food Allergy Research and Education), Elijah-Alavi Foundation, Red Sneakers for Oakley, and University of Pennsylvania Law School.[13]
Until there’s a treatment for Celiac Disease other than a gluten-free diet, our research and lived experience has informed us that requiring the labeling of gluten will have the greatest impact on improving the quality of life and consumer protection for 3.3 million American Celiacs and their loved ones.
In the spirit of Secretary Xavier Becerra sharing his mother’s wisdom at the HHS Food is Medicine Summit with “it is much better to prevent than to remediate,”[14] we hope that the Secretary will listen to the brave words of 10-year-old Jax speaking truth to power in front of 400 people at the Summit and asking our nation’s health leaders to help better protect Celiacs by requiring the labeling of gluten.[15]

Jax Bari and Admiral Rachel L. Levine, MD, HHS Assistant Secretary for Health

Jax Bari and Robert M. Califf MD, MACC, FDA Commissioner
For Additional Information:
www.celiacjourney.com/petition
[1] FALCPA amended the Federal Food, Drug, and Cosmetic Act (FDCA) as follows: Section 201 (qq) was added to define the term “major food allergen.” The term means any of the following foods, or a food ingredient that contains protein derived from any of the following foods: milk, eggs, fish, crustacean shellfish, tree nuts, wheat, peanuts, and soybeans. Section 403(w) was added to address the labeling of foods that contain a major food allergen. Effective January 1, 2006, all food labels must clearly state if food products contain any ingredients that contain protein derived from the eight major allergenic foods. See Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) (Public Law 108-282, Title II). In April 2021, the Food Allergy Safety, Treatment, Education, and Research Act of 2021 (FASTER Act) amended section 201(qq) of the FDCA to add sesame to the definition of “major food allergen.” This amendment applies to “any food that is introduced or delivered for introduction into interstate commerce on or after January 1, 2023” (Public Law 117-11).” https://www.fda.gov/media/157637/download.
[2] “Citizen Petition For The Labeling of Gluten As A Major Food Allergen,” Jonathan Bari, Leslie Bari, Jax Bari, & Lexi Bari, on behalf of Celiac Journey, September 13, 2023, FDA-2023-P-3942, https://downloads.regulations.gov/FDA-2023-P-3942-0001/attachment_1.pdf.
[3] “When Food is Poison” was written by Laura Derr when she was a student at Harvard Law School, under the supervision of Lecturer on Law Peter Barton Hutt, Partner at Covington & Burling in Washington, D.C., for Harvard Law School’s Winter 2005 Food and Drug Law course. Mr. Hutt was also former Chief Counsel to the FDA from 1971–1975. “When Food is Poison” won First Place in the 2005 H. Thomas Austern Memorial Writing Competition (long papers) sponsored by the Food and Drug Law Institute.
[4] Laura E. Derr, When Food Is Poison: The History, Consequences, and Limitations of the Food Allergen Labeling and Consumer Protection Act of 2004, 61 Food & Drug L.J 143, 143–45 (2006), https://www.jstor.org/stable/26660870.
[6] My family is grateful to Derr for writing this amazingly detailed work product memorializing the historical perspective and then-contemporaneous iterations of FALCPA. The entire article was fascinating, and the section “The FALCPA’s Impact on People with Celiac Disease” was prescient about what would become our family’s lived experience with FALCPA’s failures in adequately protecting Celiacs.
[7] When Food is Poison, Page 141, including footnotes 423–424: “See FALCPA 203(b), 21 U.S.C.A. 343(note); FALCPA 203(a), 21 U.S.C.A. 343(x). The Senate Committee Report states that it intends for any regulations issued by FDA requiring the identification of additional allergens to prescribe disclosure in ‘a manner consistent with’ the FALCPA. S. Rep. No. 108-226, at 10.” (Source: https://www.congress.gov/108/crpt/srpt226/CRPT-108srpt226.pdf). “The legislation also adds a second misbranding provision to account for other food allergens. In particular, section 403(x) provides that FDA has the authority to require by regulation appropriate labeling of any spice, flavoring, coloring, or incidental additive ingredient that is, or includes as a constituent, a food allergen that is not a major food allergen. The committee does not intend the listing of all spices or flavorings in a product but intends that the Secretary will require the food allergen to be identified on the label in a manner consistent with this legislation.” https://www.congress.gov/108/crpt/srpt226/CRPT-108srpt226.pdf.
[8] H.R. Rep. No. 108-608, at 18. (2004), https://www.congress.gov/108/crpt/hrpt608/CRPT-108hrpt608.pdf.
[9] According to the University of Nebraska-Lincoln, the following countries require that gluten be labeled on packaged foods: Anguilla, Antigua and Barbuda, Argentina, Australia, Austria, Bahamas, Barbados, Belarus, Belgium, Belize, Bermuda, Bolivia, Brazil, British Virgin Islands, Bulgaria, Canada, Cayman Island, Chile, China, Colombia, Costa Rica, Croatia, Cuba, Cyprus, Czech Republic, Denmark, Dominica, Egypt, El Salvador, Estonia, Fiji, Finland, France, Germany, Greece, Grenada, Guatemala, Guyana, Haiti, Honduras, Hong Kong, Hungary, India, Ireland, Italy, Jamaica, Kazakhstan, Kuwait, Latvia, Lithuania, Luxembourg, Malawi, Malaysia, Malta, Mexico, Montserrat, Morocco, Netherlands, New Zealand, Nicaragua, Oman, Philippines, Poland, Portugal, Qatar, Romania, Russia, Saint Lucia, Saudi Arabia, Singapore, Slovakia, Slovenia, Spain, St. Kitts and Nevis, St. Vin. and Grenada, Suriname, Sweden, Thailand, Trinidad and Tobago, Turkey, Turks and Caicos Island, Ukraine, United Arab Emirates, United Kingdom, Venezuela, Vietnam, and Yemen. Source: https://farrp.unl.edu/IRChart.
[10] https://www.nytimes.com/2018/04/13/well/eat/how-can-oats-which-dont-contain-gluten-be-labeled-gluten-free.html.
[11] https://downloads.regulations.gov/FDA-2021-N-0553-1584/attachment_1.pdf, pages 130–143; https://www.celiacjourney.com/post/readout-of-white-house-meeting-on-labeling-gluten-as-a-major-food-allergen-to-protect-celiacs.
[12] https://www.fao.org/3/cb4653en/cb4653en.pdf (emphasis added).
Update Magazine
Spring 2024

 JON BARI is President of Bari Consulting Group, which provides consulting and expert witness services in various fields including e-commerce, intellectual property, information security, and social media. In addition to co-founding Celiac Journey after his son Jax was diagnosed with Celiac Disease, Jon is the inventor of two patents in single sign-on and social media technologies.
JON BARI is President of Bari Consulting Group, which provides consulting and expert witness services in various fields including e-commerce, intellectual property, information security, and social media. In addition to co-founding Celiac Journey after his son Jax was diagnosed with Celiac Disease, Jon is the inventor of two patents in single sign-on and social media technologies. LEXI BARI is a freshman at the Wharton School of the University of Pennsylvania studying Business Economics and Public Policy as well as Finance. Lexi co-founded Celiac Journey, a patient-centric advocacy group focused on telling the pediatric perspective of American Celiacs and creating opportunities for those with lived experiences, including her brother Jax, to be heard and included in the design of equitable policies that impact them.
LEXI BARI is a freshman at the Wharton School of the University of Pennsylvania studying Business Economics and Public Policy as well as Finance. Lexi co-founded Celiac Journey, a patient-centric advocacy group focused on telling the pediatric perspective of American Celiacs and creating opportunities for those with lived experiences, including her brother Jax, to be heard and included in the design of equitable policies that impact them.