
Food Labeling Regulation
An Overview from Brazil and Latin America
By Rob Rodrigues, Ana Luíza Calil & Emanuella Buzatto John Kunz
Introduction
In recent years, Brazil together with other Latin American countries have proposed significant changes in food labeling regulation, moved by the effort to enhance consumer awareness, reduce information asymmetries, and increase health consciousness/transparency in the food industry.
Latin America and the Caribbean will account for 25% of global agricultural and fisheries exports by 2028, according to a report by the Food and Agriculture Organization of the United Nations (FAO) and Organization for Economic Cooperation and Development (OECD).[1] An Endeavor-Pepsico 2021 Report[2] shows that Brazil, Chile, Mexico, and Argentina stand out in the food market and industry, including food-innovative initiatives from unicorn startups companies.
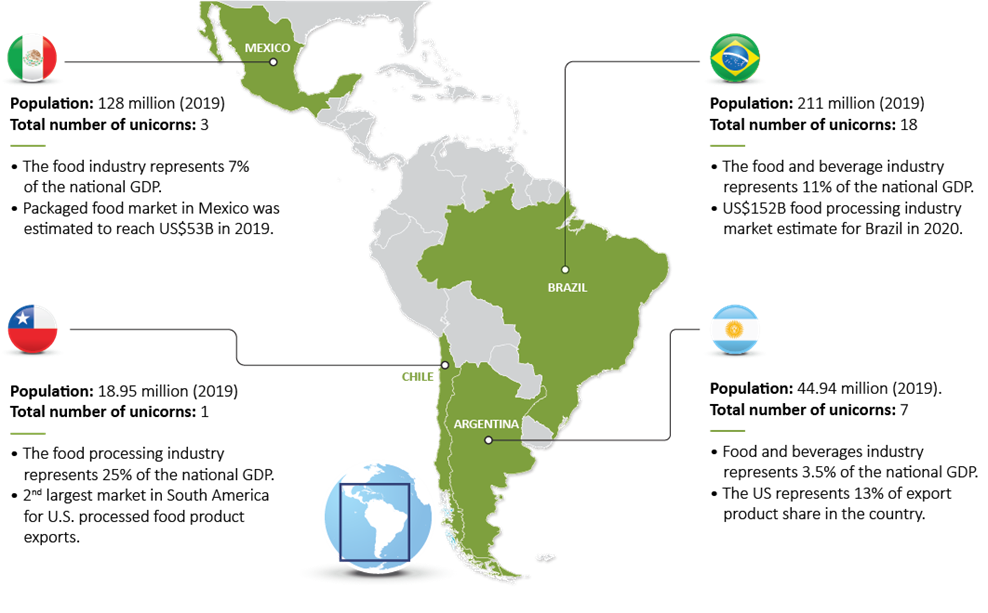
Throughout Latin America, there is a joint effort to harmonize food labeling regulations, especially within the Mercosur trade bloc (Argentina, Brazil, Paraguay, and Uruguay). The goal is to establish consistent labeling standards, facilitating trade and promoting consumer trust in the regional food supply.
As for the regulation purpose related to food labeling, nutritional transparency is the main goal in the Latin American landscape. Regulations prioritize the clear labeling of allergens, additives, and other essential information to safeguard public health and address consumer concerns. Many countries in the region align their regulatory frameworks with international standards set by organizations such as the World Health Organization (WHO) and the Pan American Health Organization (PAHO). This alignment underscores a commitment to adopt global best practices.[3]
As consumers become more health-conscious, they become more empowered to potentially shift in their purchasing behaviors. The prominence of front-of-pack warning labels and comprehensive nutritional information influences consumers’ choices, driving demand for products that meet their health criteria[4].
 Since 2017, Brazil has been conducting important changes. After three years of regulation studies, the National Food and Drug Agency (“Anvisa”) released the Board of Directors Regulation (“RDC”) #429/2020, a rule establishing front-of-pack warning labels on several products. This adds to the already-in-force nutritional labeling requirements. The new regulation was elaborated to deal with the problem, identified in the regulatory impact assessment procedure (“AIR”), that Brazilian consumers struggled to understand nutritional labels.
Since 2017, Brazil has been conducting important changes. After three years of regulation studies, the National Food and Drug Agency (“Anvisa”) released the Board of Directors Regulation (“RDC”) #429/2020, a rule establishing front-of-pack warning labels on several products. This adds to the already-in-force nutritional labeling requirements. The new regulation was elaborated to deal with the problem, identified in the regulatory impact assessment procedure (“AIR”), that Brazilian consumers struggled to understand nutritional labels.
In addition, Anvisa implemented its regulatory governance tools to simplify and consolidate regulations related to food labeling. It resulted in the publication of RDC #727/2022—the current general regulation for the matter.
Brief History: Brazilian Regulation Development in the International Scenario[5]
PHASE 1. At the international level, food labeling rules were harmonized in 1985, with the publication of the Codex Alimentarius—the first version of the Guidelines on Nutritional Labeling. In 1997, recommendations for the use of nutrition claims were agreed.
In Mercosur, the first regulation on nutritional labeling was adopted in that regulatory context. In 1994, it was agreed that nutrition labeling would be voluntary, with the exception of foods with nutrition claims. This information should be structured in tabular format and contain the amounts of energy, carbohydrates, fats, proteins, and dietary fiber, in addition to the nutrients that are the subject of nutrition claims. However, in that period, no consensus was reached on the criteria for foods with nutrition claims.
PHASE 2. On the international scene, an important change was supported by scientific reviews and dietary recommendations prepared by the WHO. In 2003, the Food Report, Nutrition and Chronic Disease Prevention reviewed the evidence for the relationship between patterns and the risk of CNCDs and concluded that the excessive consumption of certain nutrients, such as free sugars, saturated fats, trans fats, and sodium, was at the root of certain weight illnesses.
In 2004, the Global Strategy on Diet, Physical Activity and Health brought a series of recommendations to governments for the implementation of an effective strategy by promoting healthy eating and practice of physical activities. Among these recommendations was the implementation of nutritional labeling to assist consumers in making conscious food choices. These recommendations had a direct impact on Codex Alimentarius activities. Between 2006 and 2010, several labeling provisions were revised to help countries in adopting the Global Strategy.
Brazil was one of the first countries to adopt mandatory nutrition labeling as a part of a public health strategy to promote adequate and healthy food and to combat excess weight through a series of initiatives that preceded the modifications implemented by the Codex Alimentarius.
Anvisa published, in 2000, a resolution that established the mandatory nutrition labeling on packaged foods. In 2001, this regulation was revoked by two resolutions that sought to improve the rules for mandatory nutritional labeling. However, these measures have been questioned in Mercosur, as they contradicted the harmonized legislation in the bloc. As a result, negotiations began for harmonization of the matter.
In 2003, the standardization was concluded and, in 2006, its implementation was conducted by Anvisa. The Agency adopted procedures for the implementation of the nutritional labeling and incorporated a complementary Mercosur resolution, which corrected some points of the legislation on foods exempt from nutritional labeling, the values of reference for folic acid, and individual package servings.
After this period, several regulations were issued on specific matters related to the food sector, such as alcoholic beverages, fast foods, and others.
PHASE 3. As highlighted by Anvisa, this third regulatory phase has been characterized by the implementation of models of frontal nutritional labeling in addition to the declaration of the nutrition table, which are intended to communicate to consumers the main nutritional characteristics of foods in an easily visible and understandable way using different symbols on the front cover of the labels.
The importance of improving nutrition labeling and the adoption of frontal labels was also recognized in Mercosur. In 2015, the Ministers of Health signed policy recommendations and measures for the prevention and control of obesity, which include improving nutrition labeling to facilitate better decisions. In 2018, with the aim of improving the nutritional information of foods, principles for the adoption of frontal nutritional labeling were recognized by the countries that are part of the bloc.
Anvisa and Ministry of Agriculture, Livestock and Food Supply (MAPA). Anvisa and MAPA are entities that deal with attributions that are complementary. While Anvisa is responsible for, mainly, processed foods and food additives,[6] MAPA is responsible for non-industrialized vegetables (in natura), animal-origin products, and beverages.
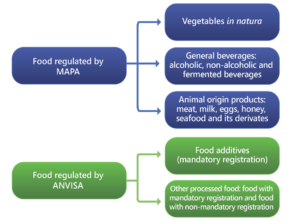
In the sphere of MAPA’s attributions, there are specific rules for labeling meat, eggs, rabbits, dairy, fish, honey, and apicultural products. It is recommended to analyze the Ministry regulations for each type of product, which have their own set of regulations, applicable not only for food labeling, but also for registration.[7]
Recent Regulatory Changes for Food Labeling in Anvisa
The table below summarizes key regulations and their effective dates. These regulatory updates align with global trends and strategies to address diet-related health concerns.
Consolidation of Food Labeling Regulations. Anvisa implemented its regulatory governance tools to simplify and consolidate regulations related to food labeling. It resulted in the publication of RDC #727/2022[8]—the current general regulation for the matter. It establishes that “label” is considered any inscription, caption, image, or descriptive or graphic matter, written, printed, stamped, engraved, embossed, lithographed, or pasted onto the food packaging.
The labeling of packaged foods must be done exclusively in the processing establishments, authorized by the competent authority of the country of origin for elaboration or fractionation. RDC #727/2022 defines each requirement for all aspects of food labeling, including details, list of ingredients, additives, and other obligatory information that shall be monitored by the company to protect the consumers.
Front-of-Pack Warning Labels. The introduction of front-of-pack warning labels in Brazil and collaborative efforts towards harmonization within the region demonstrate a commitment to improving consumer health outcomes. As consumers become better equipped to make informed decisions, the food industry is compelled to prioritize transparency, accurate labeling, and healthier product offerings. The evolving food labeling landscape reflects a region-wide dedication to enhancing consumer well-being and fostering an environment where informed choices are readily accessible.
After conducting social engagement procedures and during the regulatory impact assessment, it was identified that there were five main reasons for the need of a nutritional food labeling regulation change in Brazil:
(a) the low level of education and nutritional knowledge;
(b) the nutrition labeling model did not meet the needs of Brazilian consumers;
(c) there was a confusion about the nutrition quality caused by the nutrition labeling model;
(d) the absence of nutrition information for many foods; and
(e) flaws in the veracity of nutritional information. These causes had root causes, which related to inconsistency and gaps in the regulation in force at 2018.
Contributions received by Anvisa demonstrated that Brazilian consumers had difficulties understanding information conveyed in the labeling of food. Regarding nutritional labeling, 88% of participants indicated that the previous model did not allow an easy identification of the nutritional value of foods by the consumer, and 91% expressed the need to change the presentation of this information.
Recognizing the importance of creating a more transparent and informative food labeling, Anvisa effectively reviewed the regulation in force in 2020. Brazil now adopts the following warnings on nutritional labels:
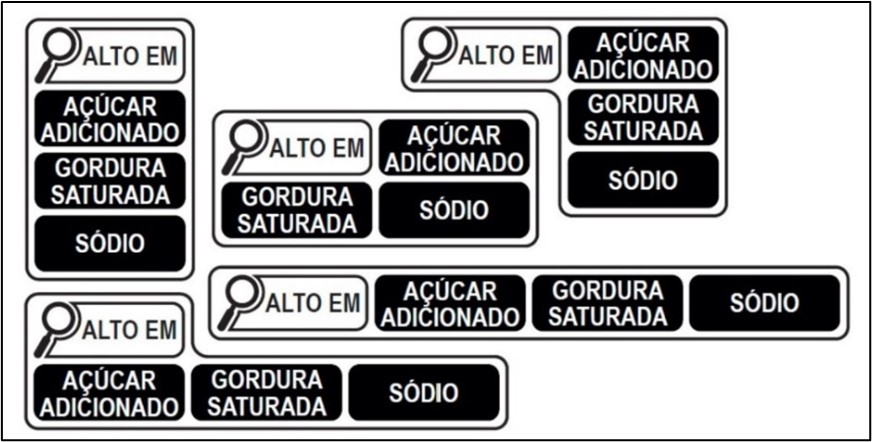
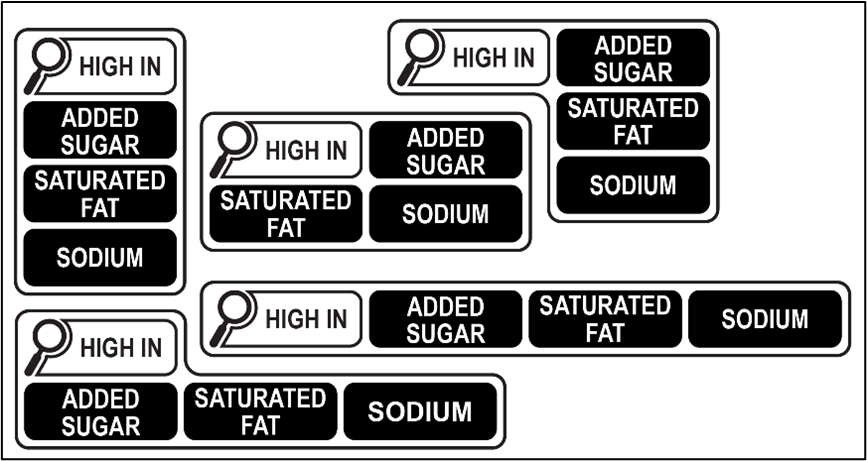
One of the notable changes was the introduction of clearer and more comprehensive nutritional information on labels. Manufacturers are now required to provide easily understandable details about calorie content, added sugars, saturated fats, and sodium levels in their products. The revised regulations include criteria for the use of nutritional symbols on the front of packages (front-of-pack labeling), aligning with the practice of other countries, such as Chile.
Obligations Imposed by RDC #429/2020
a) Front-of-Pack Warning Labels: Food products that have high quantities of specific critical nutrients, such as sugars, sodium, and saturated fats, must prominently display a front-of-pack warning label. These labels are designed with distinctive symbols and wording, delivering a clear message about the nutrient content.
b)Visual Impact: The warning labels are in the front of the package, ensuring they are easily noticeable by consumers during their purchasing decisions.
c) Specific Nutrient Thresholds: RDC #429/2020 defines specific thresholds for sugars, sodium, and saturated fats that trigger the requirement for warning labels. Products exceeding these thresholds are subject to compliance.
d) Phased Implementation: The regulation adopted a phased approach, with the first phase beginning in October 2022 and subsequent phases extending until 2025. This approach allows manufacturers to gradually adapt their labeling practices.
e) Transparency and Accountability: Manufacturers are responsible for ensuring that their products adhere to the labeling requirements outlined in RDC #429/2020. This fosters a sense of transparency and accountability within the food industry.
Monitoring. Health Surveillance Authorities (“Vigilância Sanitária” in Portuguese), operating at the local level, play a vital role in upholding and enforcing nutritional labeling regulations within their respective jurisdictions. These entities are empowered by national regulations and guidelines to ensure that food labels provide accurate and transparent information to consumers. Those entities are responsible for the following activities:
a) Compliance: Monitoring food products available in local markets, supermarkets, and other retail establishments to verify whether they comply with established nutritional labeling regulations. They ensure that labels accurately reflect the nutritional content of the products.
b) Inspection: Conducting routine inspections and audits of food manufacturers, processing facilities, and retail outlets. These inspections involve scrutinizing product labels, verifying nutrient information, and ensuring that mandatory warnings, if applicable, are correctly displayed.
c) Education: Health Surveillance Authorities play a pivotal role in educating food businesses and manufacturers about nutritional labeling requirements. They provide guidance on proper label design, accurate nutrient calculations, and adherence to specific regulations, fostering compliance within the industry.
d) Enforcement of Penalties: In cases of non-compliance with nutritional labeling regulations, Vigilância Sanitária has the authority to impose penalties, fines, or other corrective measures. This serves as a deterrent to ensure that businesses adhere to labeling standards.
e) Collaboration with Regulatory Bodies: Health Surveillance Authorities collaborate with national regulatory bodies (such as Anvisa) to be updated on the latest changes and amendments to nutritional labeling regulations. This collaboration helps them align their local efforts with broader national initiatives.
f) Monitoring Market Trends: Track of market trends, identifying emerging products or practices that might impact nutritional labeling standards. This proactive approach allows them to address potential issues before they escalate.
g) Response to Complaints: Response to consumer complaints related to misleading or inaccurate nutritional labeling. They investigate such complaints and take appropriate action to rectify any violations.
Health Surveillance Authorities are an essential part of the food regulatory system, ensuring that nutritional labeling regulations are effectively implemented at the local level.
[1] Brazilian-American Chamber of Commerce, Latin America and the Caribbean Will Account for More Than 25% of Global Agricultural Exports by 2028, Says FAO, BRAZILCHAM (July 10, 2019), https://brazilcham.com/latin-america-and-the-caribbean-will-account-for-more-than-25-of-global-agricultural-exports-by-2028-says-fao/.
[2] Endeavor Mexico, FoodTech Landscape in Latin America, PEPSICO MEXICO (2021), https://www.pepsico.com.mx/docs/librariesprovider20/sustentabilidad/endeavor-whitepaper—foodtech-landscape-in-latin-america.pdf.
[3] E-food Portal, busca por uma vida mais saudável aumenta o interesse no ‘rótulo limpo’, E-FOOD (May 21, 2021), https://portalefood.com.br/noticias/busca-por-uma-vida-mais-saudavel-aumenta-o-interesse-no-rotulo-limpo/.
[4] Unicef Technical Guidance, Front-of-Pack Nutrition Labelling: A ‘How-to’ Guide for Countries, UNICEF (2021), https://www.unicef.org/media/118716/file.
[5] This section was developed based on Anvisa’s findings in the regulatory impact assessment report regarding food labeling in Brazil, published in 2020. See Relatório de Análise de Impacto Regulatório sobre Rotulagem Nutricional (Brazilian FDA, 2019), http://antigo.anvisa.gov.br/documents/10181/3882585/%281%29Relat%C3%B3rio+de+An%C3%A1lise+de+Impacto+Regulat%C3%B3rio+sobre+Rotulagem+Nutricional/3e2c2728-b55a-4296-b5af-6c7960fd6efa.
[6] To establish a regulatory system for registration and certification of new food products, Anvisa determinates the following types of food that must have registration before commercialized: 1) food with functional properties allegations and/or health; 2) kids’ food; 3) enteral nutritional food; 4) packages with new technologies; 5) bioactives and probiotics isolated substances with functional properties allegations and/or health.
[7] Ministry of Agriculture, Cattle and Supplying, Registro de Produtos – Rotulagem, Brazilian Government (2017), https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/empresario/registro-de-produtos-rotulagem.
[8] The RDC Annex I presents the list of foods exempt from the mandatory declaration of the expiry date and Annex II brings the list of generic class names of ingredients authorized for declaration in the list of ingredients.
Update Magazine
Winter 2023

 ROB RODRIGUES has an LLM from Stanford University and is a partner at Licks Attorneys in Brazil.
ROB RODRIGUES has an LLM from Stanford University and is a partner at Licks Attorneys in Brazil. ANA LUÍZA CALIL is a professor of public law and manages regulatory disputes as a lawyer in Brazil.
ANA LUÍZA CALIL is a professor of public law and manages regulatory disputes as a lawyer in Brazil. EMANUELLA BUZATTO JOHN KUNZ is a law student and a legal intern at Licks Attorneys in Brazil.
EMANUELLA BUZATTO JOHN KUNZ is a law student and a legal intern at Licks Attorneys in Brazil.




