Deconstructing the Consent Decree: A Primer and Recent Trends for FDCA Injunctions
By Beth Weinman, Josh Oyster, and Jessica Band
The Federal Food, Drug, and Cosmetic Act (FDCA) provides authority for the government to restrain violations of the FDCA through pursuit of an injunction in federal court.[1] The Department of Justice (DOJ), which litigates on behalf of FDA,[2] routinely pursues adequately supported injunction actions upon referral from FDA to stop firms and individuals from violating the FDCA and to prevent future violations. Before filing a complaint of injunction in federal court, the government generally proposes a consent decree of permanent injunction to the would-be defendants. The consent decree is effectively a pre-negotiated injunction, many of the terms of which are non-negotiable from FDA’s perspective. If the parties can come to agreement, litigation over the terms of the injunction can be avoided, and the government files the complaint of injunction and the consent decree in federal court for judicial approval. When agreement on the consent decree cannot be reached, the government ordinarily files its complaint and asks the court to impose the government’s requested injunction.
The FDCA injunction is a powerful enforcement tool due to its broad application. Compared to a seizure,[3] which only impacts certain identified lots of violative product, an injunction, by contrast, can require a company to cease all interstate shipments of products produced under unlawful conditions. The government may seek injunctions to enforce compliance with current good manufacturing practice (cGMP) requirements, to stop the distribution of unapproved drugs or devices, or to enforce sanitation and quality requirements for food, among other contexts.[4] FDA views injunctions as important both for correcting the particular violations at issue and for serving as a deterrent to incentivize other industry participants to comply voluntarily with FDA requirements.[5]
This article describes the historical use of and current policy standards for pursuing FDCA injunctions, summarizes the typical characteristics and provisions of a consent decree of permanent injunction, and analyzes FDCA injunction cases filed since 2013 to identify key trends and takeaways for FDA-regulated entities.
Historical Use of FDCA Injunctions
Following the 1938 enactment of the FDCA, the government pursued injunctions much less frequently than the other judicial enforcement remedies available under the statute—seizures and criminal prosecutions. In the first ten years of the FDCA, 153 injunction cases were filed, as compared to 3,051 criminal prosecutions and 19,348 seizures.[6] The government pursued injunctions sparingly because they “require[d] constant policing” by FDA personnel to assure compliance.[7] One FDA attorney at the time said the agency was “loath” to use its injunction authority and would only bring injunction proceedings “after repeated prosecutions” under other FDCA provisions.[8] The injunction’s focus on controlling future behavior was viewed in this era as both “its inherent weakness” and “its unique contribution” to FDCA enforcement.[9] Yet an injunction was valuable for its collateral use with product seizures, “its application as a touchstone to guide defendant’s future conduct,” its application to guard against future “long-range” violations, and “its psychological effect as a judicial warning.”[10]
By the 1970s, the FDCA injunction was still a relatively rarely used remedy.[11] However, practitioners began to recognize the injunction as “the most severe remedy available” to FDA because of its “immediate and catastrophic effect on an enterprise which may employ hundreds or thousands of people and which literally could be wiped out overnight by the issuance of the prayed-for injunction.”[12]
Practitioners also viewed the consent decrees often used to resolve injunction matters as one-sided deals favoring the government. An FDA attorney commented in 1978 that although FDA is prepared to enter consent decrees in injunction actions, the agency “is unlikely to be willing to engage in extended negotiation” over their terms.[13] He added that FDA was unlikely to settle cases based upon “resource considerations” that might ordinarily drive a private litigant to settle.[14] Nonetheless, the consent decree was “the most prevalent and expeditious manner” of resolving an FDCA injunction case.[15] This has remained true over the last four decades.
Current Standards for FDCA Injunctions
When considering whether to refer an injunction case to DOJ, FDA evaluates several criteria, including the seriousness of the offense, the impact of the offense on the public, whether other actions could be as or more effective, and the need for prompt judicial action.[16] FDA usually favors an injunction when (1) there is a current or definite health hazard or gross consumer deception requiring immediate action; (2) a firm owns significant amounts of violative product and a voluntary recall by the firm was either refused or was “significantly inadequate” to protect the public; or (3) chronic violative practices have not (yet) produced a health hazard or consumer fraud but have not been corrected through other approaches.[17] Regarding the first two situations, FDA considers a history of prior violations and the inability to correct them through warnings or other sanctions as “helpful, but not mandatory” for bringing an injunction.[18]
In practice, the FDCA injunction continues to be used relatively rarely (see “Analysis of Recent Injunction Actions” section, below) because FDA generally seeks voluntary compliance through warning letters, recalls, and other regulatory mechanisms before pursuing judicial enforcement remedies. Just as they were in the 1940s, injunction cases today are extremely time-consuming to prepare, and successful injunctions also require significant agency resources to monitor. The government often seeks an injunction where a firm has a long history of violations and has failed to complete promised remediation.[19] Injunction actions continue to be resolved most often through a negotiated settlement—the consent decree—rather than litigation to judgment.[20]
Key Characteristics and Provisions of a Consent Decree
Although the specific provisions of a consent decree of permanent injunction will vary depending on the nature of a firm’s operations and the alleged violations, consent decrees share many common characteristics:
Identity of Defendants
At its most basic, an FDA consent decree typically involves a negotiated, court-approved order barring an FDA-regulated firm from violating the FDCA, subject to certain pre-specified remedies. When a corporate entity is involved, FDA’s policy is to also name responsible corporate officers from the firm (e.g., CEO, heads of quality or regulatory functions) as defendants.[21] The individuals to be named are frequently a matter of negotiation with the government. Identification of responsible individuals as defendants, in FDA’s view, ensures involvement by those authorized to take corrective actions and to prevent future violations, serves as a deterrent, and prevents attempts to circumvent the injunction.[22] From our review of FDCA injunctions since 2013, we identified only one case in which the government did not name at least one individual defendant.[23]
Injunctive Provisions
In most cases, a consent decree enjoins defendants from engaging in manufacturing, distribution, or other applicable activities at one or more facilities for some or all of a firm’s products, “unless and until” the defendants achieve full compliance, as determined by an independent expert and then accepted by FDA based on the consultant’s reports and the agency’s own inspection. The specific requirements for resuming operations are drafted with input from the relevant FDA product center. [24] Importantly, in most consent decrees, the defendants cannot resume enjoined operations until FDA notifies them that they appear to be in compliance. Some consent decrees, however, permit certain operations to continue, such as the manufacture and distribution of medically necessary products. The scope of the injunctive provisions, including the site(s) and product(s) affected, are generally negotiated with the government.
Periodic Auditing
Consent decrees also typically require defendants to engage an independent auditor to assess and report on their compliance at a specified frequency (e.g., annually). The auditor must deliver its reports to both FDA and the defendants. If the auditor’s reports contain any adverse observations, the defendants have a specified period of time (e.g., thirty days) to correct the issues or propose a timetable for such corrections.
“Letter Shutdown” Authority
A consent decree always includes a “letter shutdown” provision granting the government discretion to order the defendant to cease operations, conduct a recall, or take other corrective action to address violations of the decree, the FDCA, or FDA regulations. With this discretion, FDA can effectively order a defendant to shut down merely by sending a letter without needing any judicial approval. Nevertheless, publicly available information suggests that FDA invokes this broad authority sparingly.[25]
Liquidated Damages
A consent decree almost always includes a provision permitting the government to assess liquidated damages if a defendant fails to comply with the provisions of the decree, the FDCA, or FDA regulations. Typically, the defendant must pay damages to the government of (i) X dollars for each day the violation continues (where “X” may be as high as $20,000); (ii) an additional X dollars for each violation per day; and (iii) a further sum equal to the retail value (or a multiple thereof) of the violative products. Some consent decrees include a cap on the amount of liquidated damages that may be assessed in a year or in total.[26] The specific amounts and potential cap are generally negotiated with the government.
Change of Control Implications
The injunctive provisions of a consent decree typically apply to the named defendants “and each and all of their directors, officers, agents, employees, representatives, successors, assigns, attorneys, and any and all persons in active concert or participation with any of them” who have received notice of the decree.[27] In most consent decrees, a change in ownership, character, or name of the corporate defendant company must be reported to FDA prior to the change, and the defendants may be required to provide advance notice of the consent decree to any potential successor or assign. These information-sharing provisions are important because the government generally takes the position that a consent decree travels with the sale of the corporate defendant or any facility subject to the decree. For example, the government obtained a cGMP-related consent decree against Ben Venue Laboratories (BVL) in 2013. BVL’s drug manufacturing facilities were then sold to Xellia Pharmaceuticals USA (Xellia). Xellia assumed liability under the consent decree as a successor, and the court entered a modified consent decree to reflect that change.[28]
Length and Dissolution
A consent decree remains in effect until specifically dissolved or vacated by a court order. Typically, a consent decree will provide that if the defendants maintain a state of continuous compliance for at least five years either following decree entry or, if applicable, satisfaction of all obligations to resume operations under the decree, the government will not oppose a petition by the defendants for relief from the decree.
Disgorgement and Restitution
While the FDCA provides courts with jurisdiction to restrain violations of the FDCA, nowhere does it explicitly authorize the government to seek disgorgement or restitution for violations. Nonetheless, beginning in the late 1990s, the government began pursuing disgorgement or restitution[29] in certain consent decrees by arguing that they are equitable remedies that a court may impose in an equitable proceeding like an injunction case. The largest disgorgement amount ever obtained under a consent decree was $500 million from Schering-Plough in 2002.[30]
Defendants in three notable cases challenged the government’s authority to seek disgorgement or restitution but lost each time. In two of the cases, the circuit court of appeals affirmed the district court’s grant of restitution.[31] In the third case, the circuit court of appeals held that the district court had the equitable authority to order disgorgement.[32]
Despite these decisions, the government’s pursuit of disgorgement or restitution in FDCA cases remains controversial, and the government has rarely pursued either in recent years. Recent challenges to disgorgement sought by other federal agencies may provide a renewed basis to challenge the use of this remedy in the FDCA context. For example, in 2020, the Supreme Court upheld the U.S. Securities and Exchange Commission’s (SEC’s) authority to pursue disgorgement as equitable relief but imposed important limits on its use.[33] Additionally, in the October 2020 term, the Supreme Court has agreed to hear a challenge to the use of restitution in Federal Trade Commission (FTC) injunction cases.[34]
Analysis of Recent Injunction Actions (2013-Present)
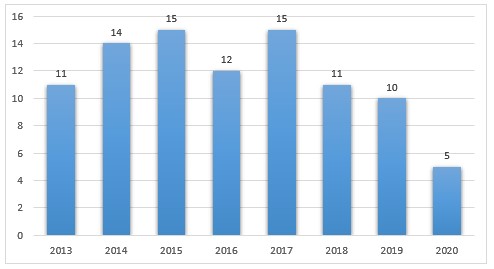
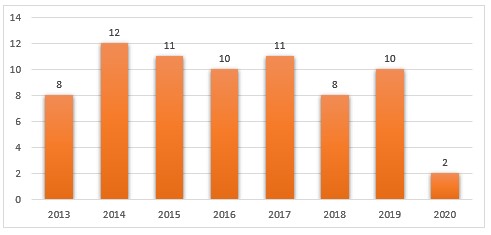
From a review of federal district court dockets and DOJ and FDA press releases, we identified ninety-three FDCA injunction cases filed between January 1, 2013 and November 15, 2020 (see Figure 1).[35] Only five FDCA injunction cases have been brought so far in 2020, likely in part due to the impact of COVID-19 on DOJ and FDA’s enforcement priorities.[36]
Figure 1. FDCA Injunction Cases (2013-Present)
Of these ninety-three cases, fifty-two (fifty-six percent) were brought against firms manufacturing or marketing foods or dietary supplements (including products purporting to be dietary supplements that the government alleged were actually drugs); sixteen involved drug manufacturers (excluding drug compounders); and thirteen involved drug compounders (see Table 1).
Table 1. FDCA Injunction Cases by Product Type (2013-Present)
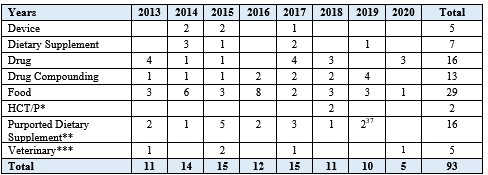
* HCT/P refers to human cells, tissues, and cellular and tissue-based products.
** The Purported Dietary Supplement category refers to cases with one or more drug-related allegations (e.g., unapproved new drug) for products that purported to be dietary supplements (as distinguished from the Dietary Supplement category for which the cases do not include any drug-related allegations).
*** The Veterinary category refers to cases involving allegations related to animal drugs, drug residues in animals, and medicated animal feeds.
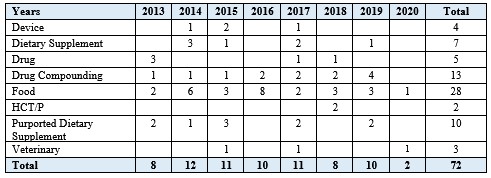
Of the ninety-three cases since 2013, seventy-two (seventy-seven percent) included cGMP or other product quality-related allegations (e.g., noncompliance with the quality system regulation (QSR) for medical devices,[38] “insanitary conditions” adulteration of foods[39] or drugs[40]) (see Figure 2).
Figure 2. FDCA Injunction Cases with cGMP or Other Quality Allegations (2013-Present)
Of the seventy-two cases with cGMP or quality-related allegations, forty-five (sixty-three percent) were brought against firms manufacturing or marketing foods or dietary supplements (including products purporting to be dietary supplements) (see Table 2).
Table 2. FDCA Injunctions with cGMP or Other Quality Allegations by Product Type (2013-Present)
From analyzing these injunction cases, we see the following trends and key takeaways:
1. Although the number of injunction actions has remained relatively consistent year to year, relatively few injunctions have been sought recently for cGMP violations by pharmaceutical and medical device manufacturers. From 2013–2019, the total number of injunction cases filed ranged from ten to fifteen per year, and the number of injunction cases with cGMP or other quality-related allegations ranged from eight to twelve per year. Despite this general consistency in case volume, the government has sought relatively few injunctions in recent years against human drug and device manufacturers for cGMP and QSR violations. Since 2013, only nine such cases have been filed (five drug and four device), and since 2016, there have been only three such cases. Instead, injunction cases have been brought primarily against food and dietary supplement manufacturers and drug compounders. These figures potentially reflect the success of FDA’s regulatory enforcement tools (e.g., inspections, warning letters, consent decrees in the early 2000s) in achieving voluntary compliance by drug and device firms without the need for injunctive relief.
2. Drug compounding continues to be a significant focus of government enforcement. Since a nationwide outbreak of fungal meningitis in 2012 caused by contaminated drugs compounded by the New England Compounding Center, DOJ and FDA have closely scrutinized the practices of drug compounders, especially those preparing sterile drugs. Since 2013, the government has pursued thirteen injunction cases against compounders (including traditional pharmacy compounders under section 503A of the FDCA, outsourcing facilities under section 503B, and animal drug compounders), and four cases were filed in 2019 alone. The focus on compounders likely reflects prioritization based on risk and the egregiousness of the violations identified in such facilities.
3. The government uses injunctions to address evolving FDA priorities like regenerative medicine. In recent years, regenerative medicine has become a significant policy and enforcement focus for FDA. In November 2017, FDA released a comprehensive framework to promote the development and approval of regenerative medicine products.[41] FDA, in conjunction with DOJ, also pursued two injunctions in 2018 against stem cell clinics, US Stem Cell Clinic and California Stem Cell Treatment Center, which allegedly offered unapproved stem cell products and failed to comply with cGMP requirements.[42] These injunctions were the first-ever brought against stem cell clinics, but they may not be the last, given FDA’s promise of “aggressive oversight” in this industry to protect patients from products that create significant potential risks and may not have been properly developed or reviewed by FDA.[43]
4. An injunction and its associated costs can have a severe adverse impact on a company’s bottom line and can even threaten its ability to survive. The effects of an injunction can be significant, especially where the injunction stops all or substantially all of a company’s operations. For example, in 2018, DOJ successfully obtained a consent decree of permanent injunction against Cantrell Drug Company, an outsourcing facility engaged in drug compounding. The consent decree prohibited the company from engaging in compounding until it had remediated past deficiencies and proved its cGMP compliance.[44] Although Cantrell successfully remediated and received notification from FDA that it could resume operations a mere five months after entry of the consent decree,[45] by that time the company had already declared bankruptcy and shut down soon after.[46] Even when a company survives a consent decree, costs associated with monitoring and ensuring compliance can be significant (in addition to any disgorgement or lost sales directly resulting from the decree). Costs typically include weighty legal and consultant fees and expenditures necessary to create the internal infrastructure to ensure compliance with the decree.[47]
- 5. The government is almost always successful in obtaining an injunction once a case is filed. Of the ninety-three injunction cases filed since 2013, the government has successfully obtained a consent decree or other order of permanent injunction in eighty-six of them. Of the remaining seven cases, five are still being litigated in district court (as of November 15, 2020); one resulted in a joint stipulation of dismissal that still required the defendant to pay equitable disgorgement;[48] and one resulted in a joint motion to dismiss after the firm’s president died, all products were destroyed, and the firm’s facility was sold.[49] These cases illustrate that once the government decides to seek an injunction, it generally has a strong case. Companies therefore should minimize the risk of injunction actions in the first instance by quickly remediating deficiencies identified during FDA inspections and internal audits.
Although the injunction is used far less frequently than many of FDA’s other enforcement mechanisms, it remains a powerful tool to stop chronic or particularly egregious misconduct and to protect the public health. The threat of a consent decree—and the significant, long-lasting obligations and burdens one imposes—is often enough to motivate FDA-regulated firms to come into compliance voluntarily.
[1] 21 U.S.C. § 332.
[2] 21 U.S.C. § 337(a); 28 U.S.C. § 516.
[3] See 21 U.S.C. § 334.
[4] DOJ, “Consumer Protection Branch: The Federal Food, Drug, and Cosmetic Act (FDCA),” https://www.justice.gov/civil/consumer-protection-branch-19 (last updated Oct. 20, 2014).
[5] See Michael R. Taylor, Seizures and Injunctions: Their Role in FDA’s Enforcement Program, 33 Food Drug Cosm. L.J. 596, 599 (1978).
[6] Lester R. Uretz, Injunction Proceedings Under the Federal Food, Drug, and Cosmetic Act, 4 Food Drug Cosm.
L.Q. 363, 373–74 (1949).
[7] Id. at 374.
[8] Edward M. Barrett, Injunction Power Under the Food, Drug, and Cosmetic Act, 5 Food Drug Cosm. L.J. 788, 790 (1950).
[9] John B. Buckley, Jr., Injunction Proceedings, 6 Food Drug Cosm. L.J. 515, 530 (1951).
[10] Id. at 531.
[11] Richard S. Morey, Handling FDA Injunction Actions, 31 Food Drug Cosm. L.J. 366, 366–67 (1976).
[12] Id. at 368.
[13] Michael R. Taylor, Seizures and Injunctions: Their Role in FDA’s Enforcement Program, 33 Food Drug Cosm. L.J. 596, 605 (1978) (“The Agency files its seizure and injunction actions on the premise that it has a sound case which will be won if litigated fully in court. Thus, if the case is to be resolved by consent decree, it must be a consent decree incorporating essentially the Agency’s terms.”).
[14] Id. at 606.
[15] David F. Weeda, FDA Seizure and Injunction Actions: Judicial Means of Protecting the Public Health, 35 Food
Drug Cosm. L.J. 112, 121 (1980) (FDA attorney remarking that all of the injunction cases during his tenure had been resolved via consent decree).
[16] FDA, Regulatory Procedures Manual, Chapter 6: Judicial Actions (“RPM”), § 6-2-4 (Apr. 2020).
[17] Id.
[18] Id.
[19] See id. § 6-2-2 (“If a firm has a history of violations, and has promised correction in the past, but has not made the corrections, the injunction is more likely to succeed.”).
[20] See, e.g., DOJ, “Principal Deputy Assistant Attorney General Benjamin C. Mizer of the Civil Division Delivers Remarks at the Food and Drug Law Institute’s Enforcement, Litigation and Compliance Conference” (Dec. 7, 2016), available at https://www.justice.gov/opa/speech/principal-deputy-assistant-attorney-general-benjamin-c-mizer-civil-division-delivers.
[21] FDA, RPM § 6-2-5.
[22] Id.
[23] Complaint, United States v. Novo Nordisk Inc., No. 1:17-cv-01820 (D.D.C. filed Sept. 5, 2017).
[24] RPM § 6-2-13.
[25] We are aware of fewer than ten publicized instances in the past two decades in which FDA invoked its authority under a consent decree to order a full or partial shutdown, a recall, or other corrective action.
[26] See, e.g., Consent Decree of Permanent Inj., United States v. Pharmedium Servs., LLC, No. 1:19-cv-03382 at 24 (N.D. Ill. May 22, 2019) ($20 million cap on liquidated damages per calendar year); Consent Decree of Permanent Inj., United States v. Aegerion Pharmaceuticals, Inc., No. 1:17-cv-11818-MLW at 25 (D. Mass. March 20, 2019) ($1 million cap on total liquidated damages).
[27] See, e.g., Consent Decree of Permanent Inj., United States v. Cantrell Drug Co., No. 4:18-cv-00159-KGB at 4, 13 (E.D. Ark. April 19, 2018) (emphasis added).
[28] Modified Consent Decree of Permanent Inj., United States v. Ben Venue Laboratories, Inc., No. 1:13-cv-00154 (N.D. Ohio April 22, 2016).
[29] Although these two terms have different meanings in different contexts, FDA has generally used the term “disgorgement” to refer to a defendant’s ill-gotten gains paid to the government, whereas FDA uses the term “restitution” when the money is returned to the purchaser. Jeffrey N. Gibbs & John R. Fleder, Can FDA Seek Restitution or Disgorgement?, 58(2) Food & Drug L.J. 129, 130 (2003).
[30] Consent Decree of Permanent Inj., United States v. Schering-Plough Corp., No. C-02-2397 (JAP) (D.N.J. May 20, 2002).
[31] See United States v. Lane Labs-USA, Inc., 427 F.3d 219 (3d Cir. 2005); United States v. Universal Mgmt. Servs., 191 F.3d 750 (6th Cir. 1999).
[32] See United States v. RX Depot, Inc., 438 F.3d 1052 (10th Cir. 2006).
[33] Liu v. SEC, 140 S. Ct. 1936 (June 22, 2020).
[34] See AMG Cap’l Mgmt. v. FTC, No. 19-508 (U.S. cert. granted July 9, 2020).
[35] To request a copy of the injunctions dataset, please contact Josh Oyster at [email protected].
[36] Beth Weinman, Josh Oyster, & Meighan Parker, Keeping Track of the Quacks: Drug and Device Enforcement in the COVID-19 Era, FDLI Update (Fall 2020).
[37] One of these cases included an allegation related to a medical device in addition to multiple allegations related to purported dietary supplements. See Compl. for Permanent Inj., United States v. Basic Reset, No. 3:19-cv-00752 (M.D. Tenn. Aug. 26, 2019).
[38] See 21 U.S.C. §§ 351(h), 360j(f)(1)(A); 21 C.F.R. Part 820.
[39] See 21 U.S.C. § 342(a)(4).
[40] See 21 U.S.C. § 351(a)(2)(A).
[41] See, e.g., FDA, “Framework for the Regulation of Regenerative Medicine Products,” https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/framework-regulation-regenerative-medicine-products (last updated May 21, 2019).
[42] See, e.g., FDA, “Statement on Stem Cell Clinic Permanent Injunction and FDA’s Ongoing Efforts to Protect Patients from Risks of Unapproved Products” (June 25, 2019), available at https://www.fda.gov/news-events/press-announcements/statement-stem-cell-clinic-permanent-injunction-and-fdas-ongoing-efforts-protect-patients-risks.
[43] Id.
[44] Consent Decree of Permanent Inj., United States v. Cantrell Drug Co., No. 4:18-cv-00159-KGB (E.D. Ark. Apr. 19, 2018).
[45] Letter from John W. Diehl (Director, Compliance Branch, Office of Pharmaceutical Quality Operations, Division II) to Dell McCarley (CEO, Cantrell Drug Company) (Sept. 21, 2018).
[46] See “Cantrell Drug Seeks Buyer,” Arkansas Business (Feb. 11, 2019) (noting Cantrell “closed its doors” in November 2018), available at https://www.arkansasbusiness.com/article/125480/cantrell-drug-seeks-buyer.
[47] See, e.g., Genzyme Co., 2010 Annual Report (Form 10-K) 20 (Mar. 1, 2011).
[48] Joint Stipulation for Dismissal and [Proposed] Order, United States v. Novo Nordisk Inc., No. 1:17-cv-01820 (D.D.C. Sept. 5, 2017).
[49] Order, United States v. Sage Pharmaceuticals, Inc., No. 5:13-cv-01983 (W.D. La. Aug. 7, 2014).
Update Magazine
Winter 2020

 BETH WEINMAN is counsel with Ropes & Gray LLP and is a member of the firm’s FDA regulatory practice group. She focuses her practice on FDA regulation and enforcement of laws governing pharmaceuticals, biologics, medical devices, and dietary supplements.
BETH WEINMAN is counsel with Ropes & Gray LLP and is a member of the firm’s FDA regulatory practice group. She focuses her practice on FDA regulation and enforcement of laws governing pharmaceuticals, biologics, medical devices, and dietary supplements. JOSH OYSTER is an associate with Ropes & Gray LLP. His practice focuses on a wide range of regulatory and compliance issues faced by life sciences and health care companies regulated under the Federal Food, Drug, and Cosmetic Act (FDCA) and related laws.
JOSH OYSTER is an associate with Ropes & Gray LLP. His practice focuses on a wide range of regulatory and compliance issues faced by life sciences and health care companies regulated under the Federal Food, Drug, and Cosmetic Act (FDCA) and related laws. JESSICA BAND is an associate with Ropes & Gray LLP’s life sciences and health care practice groups. She provides regulatory advice to life sciences companies and health care organizations on a wide range of issues focusing on FDA and health care regulatory matters.
JESSICA BAND is an associate with Ropes & Gray LLP’s life sciences and health care practice groups. She provides regulatory advice to life sciences companies and health care organizations on a wide range of issues focusing on FDA and health care regulatory matters.