Lacks v. Thermo Fisher Scientific Inc.—An Extraordinary Event from More than 70 Years Ago That Led to a Lawsuit, Resulting in a Settlement
By Véronique Li
The recent settlement agreement between Ron L. Lacks, grandson of Henrietta Lacks and executor of Ms. Lacks’ estate, and Thermo Fisher Scientific Inc., one of the largest life science companies in the world, came about after tissue from Ms. Lacks was surgically removed more than 70 years ago without her knowledge or consent.
Would this unprecedented settlement have happened if not for the publicity generated by Rebecca Skloot and her 2010 best-selling book The Immoral Life of Henrietta Lacks,[1] which became the subject behind the 2017 HBO adaptation starring Oprah Winfrey? And what does the complaint, filed by the estate of Ms. Lacks, mean for other entities that have similarly benefited from the HeLa cell line, so named using the first letters of Ms. Lacks’ first and last names? What are the broader implications of this case? We explore these questions below.
Henrietta Lacks
Henrietta Lacks was born Loretta Pleasant on August 1, 1920.[2] As a young child, Ms. Lacks worked as a tobacco farmer and cared for animals and the garden. When she was in the sixth grade, she dropped out of school to help support her family.
After her mother’s passing, she moved to live with her paternal grandfather. She eventually married David “Day” Lacks in 1941 and moved to Turner Station, Maryland, where the couple had five children.[3]
Months after giving birth to her fifth child, she felt a “painful knot in her cervix” and experienced vaginal bleeding. She went to Johns Hopkins Hospital, which was one of the few hospitals that would treat Black patients—although only in racially segregated wards.
At the time when Ms. Lacks was referred to Johns Hopkins, the chair of gynecology at the hospital, Dr. Richard Wesley TeLinde, faced criticism for frequently removing the cervix, uterus, and portions of the vagina of patients with carcinoma in situ. If he could demonstrate that carcinoma in situ behaved the way other forms of cervical cancer did, he believed he could justify his aggressive surgical techniques.
He recruited Dr. George Gey, head of tissue research at Johns Hopkins, to use samples that Dr. TeLinde would provide. Dr. Gey would then attempt to grow cells that could survive in a laboratory. The proposal aligned with Dr. Gey’s research interests to understand how human cell samples could survive in laboratory conditions.
Dr. TeLinde would go on to direct other doctors to take samples from Black patients with cervical cancer in Johns Hopkins’ segregated wards.
Treatment
On February 5, 1951, Dr. Jones took a biopsy of Ms. Lacks’ cervix and discovered a large, malignant tumor, which, upon examination, was different from any tumor he had ever seen before.
Following the biopsy and diagnosis of cancer, Ms. Lacks began undergoing treatment with radium tube inserts. Such treatment required a patient to be placed under anesthesia. It also left Ms. Lacks infertile. When she found out about her infertility, she stated she would never have agreed to be treated.[4] The treatment did not slow her cervical cancer, which she would succumb to on October 4, 1951. (As an aside, the complaint was dated October 4, 2021, marking the 70th anniversary of her passing. October 4 was also designated Henrietta Lacks Day in 2017 by then-Baltimore, Maryland Mayor Catherine Pugh.)
During one of the treatment sessions and while under anesthesia, two parts of Ms. Lacks’ cervix were removed without her knowledge or permission. Removing the tissue samples was neither medically necessary nor germane to radium treatment.
Discovery
The tissue samples were then sent to Dr. Gey’s tissue lab. He discovered that Ms. Lacks’ cells were “immortal” (i.e., cells did not die after a few cell divisions). This cell line, which would eventually become known as HeLa cells, was the first to reproduce indefinitely. In contrast, samples obtained from other patients would typically only survive for a few days.
The novelty of HeLa cells were used for many experiments and led to medical breakthroughs such as the polio vaccine, gene mapping, and in vitro fertilization. HeLa cells were also employed to understand the effects of radiation on human cells and cited in over 110,000 scientific publications.[5]
It also resulted in the first known human biological materials ever bought and sold. “One scientist estimates that if you could pile all HeLa cells ever grown on a scale, they’d weigh more than 50 million metric tons.”[6] That is a staggering amount, reflecting the outsized impact on scientific and medical research and development and subsequent profits from Ms. Lacks’ cells.
Ms. Lacks’ family did not learn about her “immortal cells” until more than 25 years after her death. Her family received no profits from the selling of HeLa cells.
Informed Consent
The standard procedure during Ms. Lacks’ time did not require a doctor to obtain consent or inform the patient when cells or tissue were taken. In fact, it was common practice to collect tissue samples from cervical cancer patients without them knowing (especially at Johns Hopkins) because of a lack of established practice in the 1950s.
As summarized in the table below, the current practice of informed consent very much differs from the practice in the 1950s.
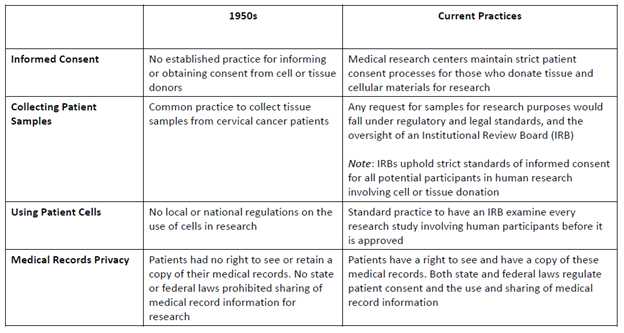
Table 1 High-Level Comparison of Clinical Research Processes[7]
In sum, the taking and subsequent use of Ms. Lacks’ tissue would not pass muster under today’s standards.
The manner in which human subjects consent (either electronically, verbally, or manually) can vary as does the contents of the informed consent itself. It is now expected that the informed consent form will explain what might happen to human tissue and how long it will be stored. This also includes specifying whether tissue, collected during the course of a clinical trial or other procedure, might be used in the future for research and what this future research might entail.
The general requirements for informed consent in U.S. Food and Drug Administration-regulated clinical research are outlined in 21 C.F.R. § 50.20. Of note: “Except as provided in §§ 50.23 and 50.24, no investigator may involve a human being as a subject in research covered by these regulations unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative.” The U.S. Department of Health and Human Services also has broader regulations for the protection of human subjects in research that require informed consent of the research subject or the subject’s legal representative (see 45 C.F.R. Part 46).
The removal of Ms. Lacks’ cells predates the development of informed consent regulations. At the time of the removal of Ms. Lacks’ cells, Thermo Fisher Scientific did not exist. The conflict that existed at the time of the procedure was with Johns Hopkins and the doctors. They were the ones who stood to benefit.
Complaint
The complaint filed by the estate of Henrietta Lacks alleged that Thermo Fisher Scientific had acknowledged publicly that Ms. Lacks’ cells were taken from her body without her consent. The complaint further stated that the estate of Ms. Lacks neither provided permission for use of her cells nor was contacted about it.
According to the lawsuit, Thermo Fisher Scientific then mass-produced HeLa cells for commercial research use and received millions of dollars in profit as a result. They also capitalized on “contract development and manufacturing services to other biotechnology companies.”[8] The plaintiff alleged the company commercialized and profited off HeLa cells without consent of or compensation to the estate of Ms. Lacks.
The sole count and single cause of action in this case is that of unjust enrichment. The estate of Ms. Lacks complained that Thermo Fisher Scientific profited from the unlawful conduct of Ms. Lacks’ doctors at Johns Hopkins. As stated in the Third Restatement of Restitution, “a defendant who is enriched by misconduct and who acts [] with knowledge of the underlying wrong to the claimant” is a conscious wrongdoer liable for its profits.[9]
The complaint goes on to claim that the company was “unjustly enriched because it received a benefit from Henrietta Lacks, understood it received a benefit from Ms. Lacks, and did so in circumstances in which acceptance or retention of the benefit was inequitable without payment or permission.”[10] Acceptance or retention of the HeLa cell line is further considered inequitable without payment or permission “through breach of a relation of trust and confidence,” “unlawful conduct,” and “because of the totality of circumstances surrounding the creation and acquisition of the HeLa cell line.”[11]
Because Thermo Fisher Scientific allegedly knew of the underlying wrong to Ms. Lacks, the estate of Ms. Lacks contends that Thermo Fisher Scientific is “liable for their net profits incurred as a result of their unjust enrichment.”[12]
Impact of Settlement
The complaint was brought forth as a result of an action that occurred more than 70 years prior. There was no dispute that Ms. Lacks’ cells were taken from her without her knowledge or consent by individuals who should have had her best interest at heart. In the ensuing 70 years, Ms. Lacks’ immortal cells were researched, patented, and commercialized over and over again.
As a result, individuals and companies have repeatedly and significantly benefited from the conduct that stemmed from the actions of doctors at Johns Hopkins. The complaint asserted that Thermo Fisher Scientific was aware of the conduct that led to the acquisition of Ms. Lacks’ cells. Their website recognized the “unsanctioned use of HeLa cells from Henrietta Lacks.”[13]
This kind of recognition, along with those of other companies, was the focus of the complaint against Thermo Fisher Scientific and a subsequent one against Ultragenyx Pharmaceutical, Inc.
Lacks v. Ultragenyx Pharmaceutical, Inc.
Thermo Fisher Scientific was the first company to be sued by the estate of Ms. Lacks. It was not the last.
On August 10, 2023, the estate of Ms. Lacks filed a suit against Ultragenyx Pharmaceutical Inc., a biopharmaceutical corporation for the same single cause of action—unjust enrichment.[14] According to its corporate presentation from August 2023, Ultragenyx’s portfolio includes four approved therapies for the treatment of rare diseases. The company’s stock symbol goes by RARE.
“The first human cells that could survive indefinitely in laboratory conditions,” the HeLa cells have allegedly been developed and mass-produced by Ultragenyx for commercial research and therapeutic use.[15] According to the complaint, profits from these activities would not have been possible without the HeLa cells.
Their proprietary HeLa Producer Cell Line (PCL) platform allegedly allows Ultragenyx to also profit from licenses and partnerships. The estate alleges that the company knowingly participates in efforts that allow the company to be compensated for the sale of products and services that are affiliated with tissue from Ms. Lacks.
Given the ubiquity of the HeLa cell lines in the biopharmaceutical industry, it may be that other companies would also potentially be targets for future claims by the estate. Thus, the implications for other research and use from biological materials across the industry may be great. While the HeLa cell line is perhaps the most famous, there were undoubtedly other tissues and biological materials that were removed from individuals decades ago without meeting current standards of informed consent. That raises the issue of possible vulnerability of other companies to these kinds of claims. It remains to be seen whether these types of risks will need to be disclosed in SEC filings or become the subject of due diligence investigations.
Publicity
It is hard to understate the role that publicity has played in this matter. What happened to Ms. Lacks received widespread attention after it became the subject of a book and movie and was referenced numerous times in scientific articles. This spotlight undoubtedly prompted the litigation, but it does not form the basis for the legal claims. Similar claims presumably could be grounded in the treatment of other individuals.
One should avoid the temptation to paint the narrative in stark terms as simply good and bad, judged solely by modern standards. The widespread use of the HeLa cell line undoubtedly led to major innovations in basic scientific knowledge and important medical breakthroughs. Still, individuals and companies have publicly acknowledged that they never sought or received permission from Ms. Lacks or her estate, yet published, manufactured, and licensed the HeLa cells. Her doctors should have focused on treating her cancer and providing the best patient outcomes. However, they improperly extracted from her cells that would ultimately prove to be reproducible.
Lessons
The case is important. It highlights what can happen without informed consent (namely that genetic material can be removed from a person without their knowledge or permission), what then happens to the genetic material (in this case, it was harvested and used numerous times in scientific and medical research and development for personal and corporate gains), who benefits from use of personal material (researchers, doctors, international companies, and patients but the individual providing the tissue), and potential legal risks to other entities who have and continue to use the HeLa cell line. That Ms. Lacks herself stated she would not have consented poses an ethical quandary for those who knowingly continue to use her cells for commercial and research purposes.
*****
Admittedly, it is disconcerting to learn that one person’s cellular materials can be repurposed for financial gains for all those but her and her family. However, in this digital age, we also have to consider privacy infringement. How much of our digital DNA is lurking in the ether for those to exploit and monetize? While it remains to be seen what will happen in the case against Ultragenyx and possibly other companies, we do have to consider whether our informed consent is necessary for other activities. Just how much do we permit our own information or material to be used when we sign, whether electronically or manually, waivers and authorizations, informed consent forms, and other documents that are long, complex, and often never read? Do we as participants have the right to benefit from any commercial products, partnerships, and licenses? It’s something to think about the next time you are presented with text-heavy documents in which you agree to give away rights to your own personal information and tissue.
[1] Rebecca Skloot, The Immortal Life of Henrietta Lacks (2010).
[2] Id. at 18.
[3] The Amazing Henrietta Lacks, Henrietta Lacks Legacy Group, https://henriettalackslegacygroup.org/the-amazing-henrietta-lacks/about-henrietta/ (last accessed Aug. 28, 2023).
[4] Compl., Lacks v. Thermo Fisher Scientific, Inc., No. 1:21-cv-02524 (D. Md. Oct. 4, 2021), EFC No. 1 [hereinafter Thermo Fisher Scientific Complaint].
[5] Amanda Holpuch, Family of Henrietta Lacks Settles With Biotech Company That Used Her Cells, N.Y. Times (Aug. 1, 2023), https://www.nytimes.com/2023/08/01/science/henrietta-lacks-cells-lawsuit-settlement.html.
[6] Skloot, supra note 1.
[7] Upholding the Highest Bioethical Standards, Johns Hopkins Medicine, https://www.hopkinsmedicine.org/henrietta-lacks/upholding-the-highest-bioethical-standards (last accessed Aug. 28, 2023).
[8] Thermo Fisher Scientific Complaint, supra note 4, at 12.
[9] Restatement (Third) of Restitution and Unjust Enrichment § 51(3) (2011).
[10] Thermo Fisher Scientific Complaint, supra note 4, at 12.
[11] Id. at 13.
[12] Thermo Fisher Scientific Complaint, supra note 4, at 13.
[13] Amanda Maxwell, Achieving Equitable Minority Representation in Biobanking, Thermo Fisher Scientific (Mar. 14, 2017), https://www.thermofisher.com/blog/biobanking/achieving-equitable-minority-representation-in-biobanking/.
[14] Compl., Lacks v. Ultragenyx Pharmaceutical, Inc., No. 1:23-cv-02171 (D. Md. Aug. 10, 2023), EFC No. 1.
[15] Thermo Fisher Scientific Complaint, supra note 4, at 9.
Update Magazine
Fall 2023

 Véronique Li, Senior Medical Device Regulatory Expert, Hyman, Phelps & McNamara, P.C. (HPM), leverages her FDA experience to provide strategic and technical guidance to clients regarding regulatory and compliance issues. In addition, she partners with the firm’s attorneys to conduct due diligence and work on government and internal investigations.
Véronique Li, Senior Medical Device Regulatory Expert, Hyman, Phelps & McNamara, P.C. (HPM), leverages her FDA experience to provide strategic and technical guidance to clients regarding regulatory and compliance issues. In addition, she partners with the firm’s attorneys to conduct due diligence and work on government and internal investigations.