The Future of Food? CRISPR-Edited Agriculture
By Abby Meyer and Sara Dastgheib-Vinarov
I. Introduction
The scientific and agricultural communities have long understood that climate variability can increase crop failures, potentially causing food shortages. More people are now recognizing that changing and more extreme weather patterns, including drier conditions in growing regions (which can make crops susceptible to disease and pests), will continue to negatively impact global food security.
To help combat growing concerns over food security, some university researchers and agri-companies are using novel gene-editing technologies to modify staple crops to make them more tolerant to the changing climate and its associated perils. Recently, scientific attention has been directed to a new molecular technique called CRISPR gene-editing technology for introducing commercially desirable traits in crops. It is believed that CRISPR can have a positive impact on food productivity, quality, and environmental sustainability. This will become more important as the global population continues to grow, and less arable land and less water resources are available to grow crops, in part due to environmental change.
This article will explore the variety of trait modifications being pursued using CRISPR technology, the diversity of the agricultural crops that are the subject of these modifications, and the key players in this space identified through patenting and licensing activity. This article will also summarize the key U.S. and European regulations affecting the labeling of food items developed using CRISPR technology and provide reasons why governmental regulatory bodies worldwide should support and harmonize CRISPR-edited agricultural guidelines.
II. What is CRISPR?
CRISPR is an acronym for Clustered Regularly Interspaced Short Palindromic Repeat, and is a relatively new genome-editing technology.[1] CRISPR technology is a powerful tool that allows researchers to more easily alter DNA sequences and modify gene function in animals or plants.
The various potential products of CRISPR technology carry the promise to contribute to solving many of the great challenges of the twenty-first century, from medical and health issues to food and agricultural production. This may certainly be one of the reasons why the 2020 Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna for their discovery and development of one of the most popular gene-editing tools: CRISPR-Cas.[2]
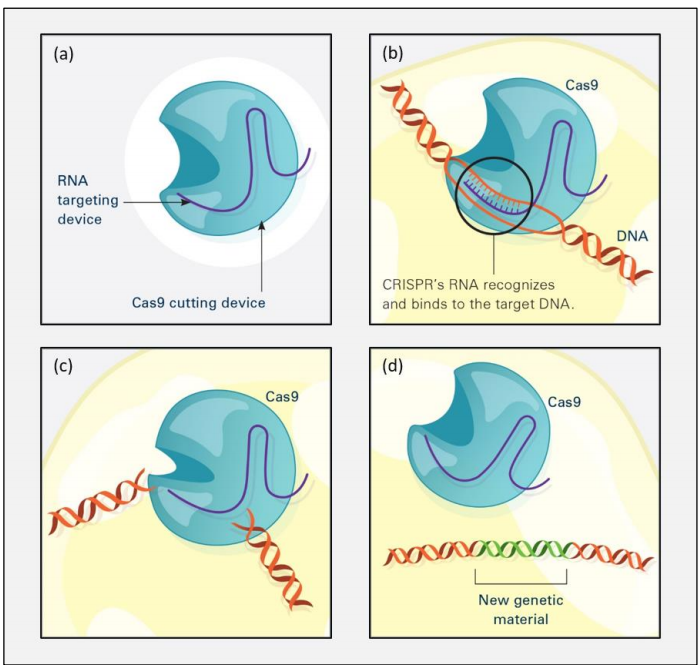
Over the years, the working principle behind CRISPR has been widely analyzed and explained in several journal reviews.[3] In general, modification of plant genomes using CRISPR gene editing technology follows the process shown below in Figure 1: (a) The CRISPR system has two components joined together: a finely tuned targeting device (a small strand of RNA programmed to bind to a specific DNA sequence) and a strong cutting device (an enzyme called Cas9 that can cut through a double strand of DNA at the binding site); (b) Once inside a cell, the CRISPR system locates the DNA it is programmed to find. The CRISPR seeking device recognizes and binds to the target DNA (circled, black); (c) The Cas9 enzyme cuts both strands of the DNA; (d) Researchers can insert into the cell new sections of DNA. The cell automatically incorporates the new DNA into the gap when it repairs the broken DNA.[4]
The CRISPR process differs from conventional GM (Genetically Modified) methods. In general, CRISPR targets pre-existing nature-derived gene variability within the crop family to bring about targeted variation and a desired output. Specifically, CRISPR is used as a set of molecular scissors to cut and delete (or replace) a gene expressing an economically undesirable trait with a nature-generated “improved” variation of the gene (i.e., existing in the plant family via natural genetic diversity). In other words, CRISPR does not use classical GM crop production techniques, which insert exogenous or foreign DNA from a vector (a vehicle which delivers foreign genetic material) into the plant genome. CRISPR enables desirable crop traits by introducing DNA from nature-generated genetic variations within the crop itself, and not from another reproductively incompatible organism. This eliminates the fear that foreign DNA may be present in the resultant plant and related finished goods.[5]
The CRISPR process’ ability not to introduce foreign DNA into the plant genome has enabled the plant products to be considered non-GMO by many scientific researchers and at least some products developed using this technology will not need to be labeled GMO under USDA rules (further discussed below). The regulatory landscape around the use of CRISPR in agriculture will be further explored later in this article.
III. Patent Landscape of CRISPR in Agriculture
It should not be surprising that the development of CRISPR’s game-changing technology was the subject of a series of patent priority, inventorship, and hence, ownership disputes between high profile research institutions. With the resolution and settlement of these disputes, there has been an increase in commercial applications of CRISPR in economically important plants and agriculture to develop desirable and improved traits.
To maximize the impact of CRISPR for improving agriculture and provide a clear path for obtaining licenses to key portions of the CRISPR intellectual property, in 2017, Pioneer-DuPont (now Corteva AgSciences) and the Broad Institute, U.S.-based holders of the two largest foundational CRISPR patent portfolios, joined forces to license CRISPR (particularly, CRISPR-Cas9) technology. Both agreed to jointly provide non-exclusive licenses for commercial use of the full suite of foundational CRISPR patents in crop agriculture. Just as important, this intellectual property became freely available for use by academic research institutions. Providing licensing opportunities has been one way to encourage scientists in both academia and industry to innovate with CRISPR and explore new ways to improve commercially desirable agricultural traits.
Generally, a good indicator of innovation in a field is the number of patent filings. To obtain an overview of the patent landscape for CRISPR-Cas technology in agriculture, we conducted a series of searches on CRISPR-Cas applications in plants over a series of several months to determine the global hotspots of R&D activity, players, plants (namely commercial crops), and traits modified. We performed a variety of search queries[6] using international public patent databases (such as WIPO [World Intellectual Property Organization], EPO [European Patent Office] and USPTO [United States Patent and Trademark Office]) to obtain search results and arrive at meaningful conclusions. As we performed the searches and evaluated the results, it became clear that the results were complicated by a number of factors. One factor was that the CRISPR tool was a recent technological breakthrough, and use of the tool to enhance traits in plants and agriculture was even more recent. It is apparent from the patent filing data that patent applications relating to use of CRISPR in commercial agriculture began around 2014–2015 and have steadily increased annually (see Figure 2).
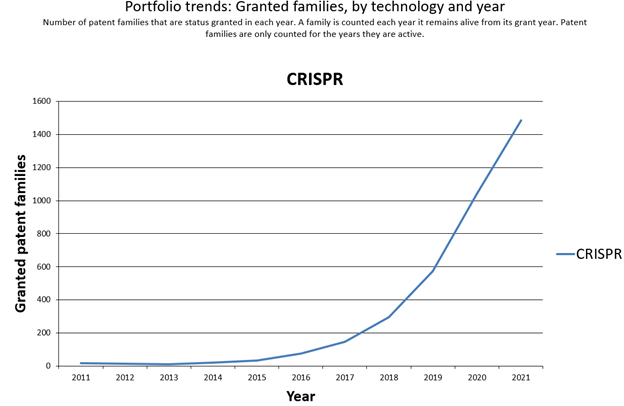
Source: Cipher[7]
Because CRISPR is new, the use of the terminology in patent filings is evolving and applicants have become their own lexicographers in describing CRISPR in applications. Specifically, we observed that applicants are defining genome editing[8] broadly in the descriptions of patent applications—listing CRISPR-Cas (without any technical support or enabled examples), along with many other gene editing tools. While the apparent intent of the applicants is to generate actual data using the CRISPR tool sometime in the future and claim priority to the earlier filing date, the effect is to create a high “noise to signal” ratio. This is because many patent applications will speculate as to using CRISPR-Cas to alter desired plant traits—without actually having done so. Another point that must be considered is that patent applications are generally published 6 to 24 months after the original filing date, depending on a variety of country-specific factors. For such a young technology, there could be many pending applications on CRISPR plant trait modifications that are not yet publicly available (i.e., unpublished). Therefore, it is expected that the patent application statistics presented here will continue to evolve.
With that backdrop, the results were not too surprising given that CRISPR technology was primarily developed through a series of independent and collaborative academic efforts from scientists in research institutions throughout North America and Europe. Notably, the search results show that the United States is dominating the commercial CRISPR agricultural patent landscape. Exemplary organizations are listed in Figure 3.
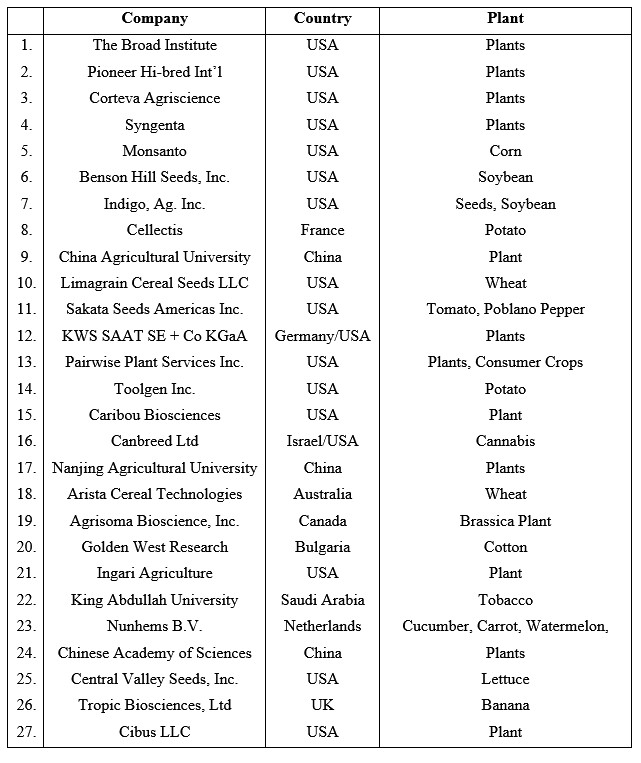
Australia and Canada appear to be second and third behind the U.S. in use of CRISPR in commercial agriculture to modify traits. The results indicate that Europe, while it has 38 European Patent Organisation member states, is behind Canada and is ahead of most Asian countries in CRISPR plant modification. However, when analyzing patent data, it is important to consider that the WIPO (international) applications, which make up at least a quarter of the patent applications, are primarily from Chinese universities or governmental research institutions. Interestingly, unlike the U.S., China, which is the most active country in academic genome editing research, has not announced the regulatory status of plant gene editing or of gene-edited foods.[9] The reasons for this are unclear, but it is possible that China wants to have robust patent protection on CRISPR modifications and a commercial product in hand before releasing regulations.
Likewise, there are relatively fewer patent filings in the CRISPR agricultural space coming out of Europe. One reason for this may be that European regulations have inhibited interest and funding for GMO agricultural products, thus impeding the progress of market-oriented agricultural applications.
The patent filings from the leading international players cover all plant types, including rice, tomato, maize, soybean, tobacco, alfalfa, wheat, rapeseed (canola), cotton, and cannabis. The United States appears to have its main patent filings in crops such as rice, tomato, maize, alfalfa, wheat, and cabbage (Brassicas). Among the CRISPR-edited plants, there are a whole host of commercially desirable traits that have been altered by patent applicants. From our patent landscape review, we observed that while some applications aim to satisfy consumer needs, others aim to benefit the farmer. Examples of consumer-focused traits include gluten free, reduction in browning, reduction in allergen levels, improved fruit color, flavor, and shelf life. Farmer-benefiting traits include viral, bacterial, fungal, temperature and drought resistance, stress and drought tolerance, and improved crop yield, among others. Such CRISPR-edited plants will enable new food products to reach the market quickly at affordable prices.
Recent examples of such products include browning-resistant mushrooms,[10] high-amylopectin waxy corn (Zea mays),[11] and false flax (Camelina sativa) with enhanced omega-3 oil.[12] These were each developed using CRISPR and approved by the U.S. Department of Agriculture (USDA) in record time.
It is not surprising that the first CRISPR-altered crops have been introduced in the United States and not Europe as the EU’s GM laws, which CRISPR-altered crops are subject to, are very tough. This is also reflected in the patent landscape—most patent activity is in North America. The relatively permissive regulatory atmosphere for CRISPR technology in the United States is why innovative agricultural companies are proactively licensing CRISPR technology and putting their agri-science expertise to work to make one-of-a-kind fruits and vegetables that can lead the way to better health for people and animals. Therefore, based on the patent landscape, it is anticipated that more CRISPR-edited crops are in the pipelines of various companies and may soon appear on the market, at least in the United States.
Regulation of GMO Agricultural Products in Europe and the United States
A. The EU’s GMO Directive and Recent Developments
In 2001, the European Parliament and Council of the European Union adopted Directive 2001/18/EC on the deliberate release of genetically modified organisms into the environment—the “GMO Directive.” The GMO Directive defines a GMO as “an organism, with the exception of human beings, in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination.”[13] The EU adopted the Directive because, among other things, it was concerned that certain GMOs could negatively impact human health and the environment, and therefore the release of GMOs needed to be controlled.
In 2003, the EU added to the GMO Directive with Regulation 1829/2003 (on genetically modified food and feed) and Regulation 1830/2003 (concerning the traceability and labeling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms). The former Regulation requires that “food and feed consisting of, containing or produced from genetically modified organisms . . . should undergo a safety assessment . . . before being placed on the market within the Community.”[14] The latter “puts in place rules to ensure products containing GMOs and food and animal feed derived from them can be traced at all stages of the production and distribution chain.”[15] It also provides that final consumer goods containing GMOs must identify the presence of GMOs on the label.
Despite providing a robust GMO regulatory framework, there remains uncertainty as to whether organisms obtained by mutagenesis are considered GMO under EU law. The Court of Justice of the European Union addressed the question in Case C-528/16 on July 25, 2018.[16] The Court explained that since the GMO Directive was adopted in 2001, technical progress in mutagenesis techniques “makes it possible to obtain the same effects as the introduction of a foreign gene into the organism (transgenesis) and those new techniques make it possible to produce genetically modified varieties at a rate out of all proportion to those resulting from the application of conventional methods of mutagenesis.” Citing the same concerns for human health as underscored the GMO Directive, the Court found that “the GMO Directive is also applicable to organisms obtained by mutagenesis techniques that have emerged since its adoption.”
Following this judgment, the Council of the European Union asked the Commission to submit a study and proposal on the status of new genomic techniques under EU law.[17] The study, issued on April 30, 2021, confirms that new genomic techniques (including CRISPR) are subject to the GMO Directive.
While concerns about the interactions of GMOs with human health and the environment remain, competing policy considerations have arisen since 2001. The April 30, 2021 study for instance notes that “the EU needs to develop innovative ways to protect harvests from pests and diseases and to consider the potential role of new innovative techniques to improve the sustainability of the food system.” The study specifically discusses CRISPR, calling it a “game-changer,” and noting that the technology is more efficient, easier to use, and more affordable when compared to previous technologies. However, the 2001 GMO Directive and 2018 Court of Justice decision appear to have stymied public and private research on new genomic techniques relating to commercial agriculture in the EU, which is reflected in the patent search results shared in this article.
The future direction and scope of the GMO Directive is at a watershed. In conjunction with issuing the April 30 study, the European Commission concluded that the Directive “is not fit for purpose for some NGTs [“novel/new genomic techniques”] and their products, and that it needs adaptation to scientific and technological progress.”[18] The Commission requested an impact assessment on a new policy initiative, to take a closer look specifically at plants derived using techniques and technologies such as CRISPR. The inception impact assessment was issued on October 22, 2021.[19] The assessment envisages policy action that will continue to protect human, animal, and environmental health, while also allowing the EU to incentivize and facilitate the development of plants, in support of both the agri-food system and the EU’s sustainability goals.[20] The Commission is targeting adoption of updated policies in 2023.[21]
B. GMO Regulation by the United States
In 1986, the United States issued its comprehensive federal regulatory policy for ensuring the safety of biotechnology products, known as the Coordinated Framework for Regulation of Biotechnology. Through the Coordinated Framework, the U.S. Department of Agriculture (USDA), Food and Drug Administration (FDA), and Environmental Protection Agency (EPA) share responsibility for regulating the products resulting from agricultural biotechnology.
USDA has several roles under the Coordinated Framework. Under the Plant Protection Act, USDA regulates products of biotechnology that may pose a risk to agricultural plant health. USDA coordinates the Biotechnology Risk Analysis Programs, which address potential plant pest risk and environmental impacts of certain genetically engineered organisms on the human environment by conducting risk and environmental assessments of these products.
In 2016, Congress passed the National Bioengineered Food Disclosure Law, which directed USDA to establish a national, mandatory standard for disclosing foods that are or may be bioengineered. USDA issued its final rule on this topic on February 19, 2019.[22] Similar to the GMO Directive, the rule applies to foods modified using techniques that “could not otherwise be obtained through conventional breeding or found in nature.” USDA refused to define the term “found in nature,” believing that “attempting to do so may cause confusion in light of the rapid pace of innovation.” Important for our purposes, some genomic editing results achieved from mutagenesis techniques like CRISPR do result in alterations that could occur naturally.[23] As a result, not all food products developed using CRISPR will be subject to this USDA final rule. The mandatory compliance date with the rule is January 1, 2022.
On May 14, 2020, USDA’s Animal and Plant Health Inspection Service (APHIS) issued its final Sustainable, Ecological, Consistent, Uniform, Responsible, Efficient (SECURE) rule, which is meant to reduce the regulatory burden for developers of organisms that are unlikely to pose plant pest risks.[24] The revised regulations evaluate whether a plant requires oversight based on the characteristics of the plant itself, and not on the method by which the plant was genetically engineered. (This is in contrast to the EU, which focuses on the process used to obtain the modification.) As to CRISPR and similar gene-editing techniques, APHIS found that the modifications obtained from them have a history of safe use with respect to plant pest risk.[25] The SECURE rule was fully implemented as of October 1, 2021.
FDA’s regulations are intended to ensure that foods (including GMO foods) pose no risk to human health. (See Federal Food, Drug, and Cosmetic Act (FFDCA), 21 U.S.C. §301 et seq.). Under the FFDCA, all food and feed manufacturers must ensure that the domestic and imported products they market are safe and properly labeled. FDA does not differentiate here between GMO and non-GMO products. The “regulatory status of a food” is instead “dependent upon objective characteristics of the food and the intended uses of the food.”[26] Additionally, FDA must approve any food additive before it can be marketed, unless the additive is “generally recognized as safe” (GRAS). FDA treats most foods derived from GMO plants as GRAS, unless the product that the plant expresses due to GMO (e.g., proteins, carbohydrates, fats) “differs significantly in structure, function or composition from substances found currently in food.”[27] As described above, some CRISPR modifications to agricultural plants are meant to increase the amount of omega-3s and other compounds presently found in the plants; these may well continue to be considered GRAS under an existing FDA policy stating that “when the substance present in the food is one that is already present at generally comparable or greater levels in currently consumed foods, there is unlikely to be a safety question sufficient to call into question the presumed GRAS status of such naturally occurring substances.”[28]
Finally, EPA registers and approves the use of all plant pesticides, including those incorporated through genetic engineering (i.e., plant-incorporated protectants, or “PIPs”), under the Federal Insecticide, Fungicide, and Rodenticide Act (7 U.S.C. § 136 et seq.). EPA uses this process to determine a PIP’s environmental safety.
While USDA, FDA, and EPA have periodically updated their policies since 1986, there is some question of whether the Coordinated Framework can sufficiently regulate the products of new mutagenesis (and other) technologies and the labeling of the resultant food products.[29] It has been noted, for instance, that GMOs that confer improved nutritional qualities and resistance to environmental stress (e.g., drought) may challenge existing regulatory processes.[30]
V. Conclusions
Although there are various hurdles to overcome in different jurisdictions to produce CRISPR-altered crops and to make them widely available in the grocery store, CRISPR is likely to revolutionize how we eat. It is wise to closely monitor both the patent and regulatory landscapes. It will be interesting to see whether the European Commission’s anticipated new policies in 2023 reach the same conclusion as the United States, reflected in the SECURE rule. Such a finding by the EU, and resulting policy changes, could lead to more R&D investment and innovation within the EU, possibly bringing the level of investment to a par with the United States.
Domestically, it will also be interesting to see to what extent CRISPR modifications challenge the Coordinated Framework and existing policies. For instance, where will FDA draw the line between new agricultural products that express more of a protein, carbohydrate, fat, or oil, and those that express a substance that differs too significantly, requiring that new food to undergo premarket approval as a food additive.
For researchers who would develop modified agricultural products using CRISPR, and for food manufacturers who would include these products into food items, the regulatory variance between U.S., EU, and other nations will be something to continue to watch and monitor. More than 60 countries require some form of GMO labeling, and differences exist as to the types of foods that must be labeled, the threshold of GMO content above which foods must be labeled, and the manner in which foods must be labeled. Countries also differ in their requirements for standards, testing, certification, and enforcement.[31] In part, this is because regulatory policy cannot keep pace with the fast-moving scientific advances, new technologies do not fit into old regulatory definitions and paradigms, there is difficulty in international coordination, lack of harmonized definitions and laws, lack of public understanding and trust, and little political will. Further, regulatory and policy officials are frequently tasked with the sometimes conflicting goals of ensuring public and environmental safety while addressing public perception and expectations and doing so without impeding innovation. Regardless, it is hoped that these insights will spur action and lead to global enhanced coordination and harmonization of regulatory processes and policies around use of CRISPR technology to develop market-oriented agricultural traits. This will help to improve the organoleptic properties of food, reduce food waste, and advance the global sustainability efforts of modern agriculture.
[1] Zaidi SS, et al. New plant breeding technologies for food security. Science. 2019;363(6434):1390–1.
[2] https://www.nobelprize.org/prizes/chemistry/2020/summary/.
[3] Chen K, et al. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol. 2019;70:667–97; Hanna RE, Doench JG. Design and analysis of CRISPR-Cas experiments. Nat Biotechnol. 2020;38(7):813–23.
[4] Figure and figure notes adapted by CRS from National Institutes of Health, “Gene Editing—Digital
Media Kit,” at https://www.nih.gov/news-events/gene-editing-digital-press-kit.
[5] He Y, Zhao Y. Technological breakthroughs in generating transgene-free and genetically stable CRISPR-edited plants. aBIOTECH. 2019;1(1):88–96.
[6] Sample search queries – ALLTXT:(CRISPR and CAS9 and trait and plant and food or agri* or crop*) and/or ACLM/(CRISPR and CAS9 and trait and plant).
[7] CIPHER is an intellectual property analytics tool for the business and academic communities. Its mission is to aggregate, analyze, and visualize data relating to patents and related events including litigation and licensing.
[8] Sample generic disclosure in patent applications: Genome editing. A type of genetic engineering in which DNA is inserted, replaced, modified, or removed from a genome using artificially engineered nucleases or other targeted changes using homologous recombination. Examples include, but are not limited to, use of zinc finger nucleases (ZFNs), TAL effector nucleases (TALENs), endonucleases, meganucleases, CRISPR/Cas9, and other CRISPR related technologies. Ma et. al., Molecular Plant, 9:961-974 (2016); Belhaj et. al., Current Opinion in Biotechnology, 32:76-84 (2015).
[9] Although China does regulate other aspects of GM crops. See, e.g., USDA Foreign Agricultural Service Gain Report Number CH 18085, available at https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Agricultural%20Biotechnology%20Annual_Beijing_China%20-%20Peoples%20Republic%20of_2-22-2019.pdf.
[10] Waltz E. Gene-edited CRISPR mushroom escapes US regulation. Nature. 2016;532(7599):293.
[11] Waltz E. CRISPR-edited crops free to enter market, skip regulation. Nat Biotechnol. 2016;34(6):582.
[12] Waltz E. With a free pass, CRISPR-edited plants reach market in record time. Nat Biotechnol. 2018;36(1):6–7.
[13] https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32001L0018; see also https://ec.europa.eu/food/plants/genetically-modified-organisms/gmo-legislation_en.
[14] https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32003R1829.
[15] https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=LEGISSUM:l21170.
[16] Organisms obtained by mutagenesis are GMOs and are, in principle, subject to the obligations laid down by the GMO Directive (europa.eu).
[17] gmo_mod-bio_ngt_eu-study.pdf (europa.eu).
[18] gmo_mod-bio_ngt_letter.pdf (europa.eu).
[19] https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-plants-produced-by-certain-new-genomic-techniques_en.
[20] Id.
[21] Id.
[22] 83 Fed. Reg. 65814, available at https://www.federalregister.gov/documents/2018/12/21/2018-27283/national-bioengineered-food-disclosure-standard#sectno-reference-66.3.
[23] “The Status under EU Law of Organisms Developed through Novel Genomic Techniques,” available at https://www.cambridge.org/core/journals/european-journal-of-risk-regulation/article/status-under-eu-law-of-organisms-developed-through-novel-genomic-techniques/4812A77647B94B3BB789D3532379C081.
[24] BRS_2020518.pdf (usda.gov).
[25] Id. at 29793.
[26] FDA, “Statement of Policy – Foods Derived from New Plant Varieties,” 57 Fed. Reg. 22984, May 29, 1992.
[27] Id. at 22990.
[28] Id.
[29] Congressional Research Service, “Agricultural Biotechnology: Overview, Regulation, and Selected Policy Issues,” available at https://crsreports.congress.gov/product/pdf/R/R46737.
[30] Id.
[31] Id.
Update Magazine
Winter 2021

 ABBY MEYER is a partner in the Business Trial Practice Group and the co-lead for the firm’s Food & Beverage team at Sheppard, Mullin, Richter & Hampton LLP. She is also a member of the firm’s Class Action Defense, Cannabis, and Healthcare teams. Abby earned her BA from the University of Southern California and her law degree from the University of California, Los Angeles School of Law.
ABBY MEYER is a partner in the Business Trial Practice Group and the co-lead for the firm’s Food & Beverage team at Sheppard, Mullin, Richter & Hampton LLP. She is also a member of the firm’s Class Action Defense, Cannabis, and Healthcare teams. Abby earned her BA from the University of Southern California and her law degree from the University of California, Los Angeles School of Law. SARA DASTGHEIB-VINAROV is Global Legal Head of Intellectual Property, Tate & Lyle Americas LLC. Sara earned a BS in Biochemistry from Trinity University, a PhD in Biochemistry from the University of Notre Dame, and a law degree from Marquette University.
SARA DASTGHEIB-VINAROV is Global Legal Head of Intellectual Property, Tate & Lyle Americas LLC. Sara earned a BS in Biochemistry from Trinity University, a PhD in Biochemistry from the University of Notre Dame, and a law degree from Marquette University.