FDCA Statutory Supplement, 2021 (2nd Edition)
Edited by Emily K. Strunk, William A. McConagha, Jennifer L. Bragg, and Deborah M. Shelton
Details
Softbound | 700 pages
Print ISBN | 978-1-935065-93-7
E-Book ISBN | 978-1-935065-94-4
Overview
The FDCA Statutory Supplement, 2021 (2nd Edition) is an indispensable tool for FDA law practitioners. This publication provides cross-referencing of the numerous legislative amendments with the original statute, facilitating quick research and citation, and highlights recent changes through simple formatting. The appendix contains relevant portions of related statutes. This edition is updated to incorporate pertinent sections of the CARES Act and other statutory amendments since the 2018 edition. This publication is available in both print and e-book versions.
Please note that we are experiencing shipment delays of our printed texts due to COVID-19. We encourage individuals to consider our e-book options, which are usually available within one business day.
Purchase
$129
Membership or Proof of Class Required | +$30 to add Print or E-Book at Checkout
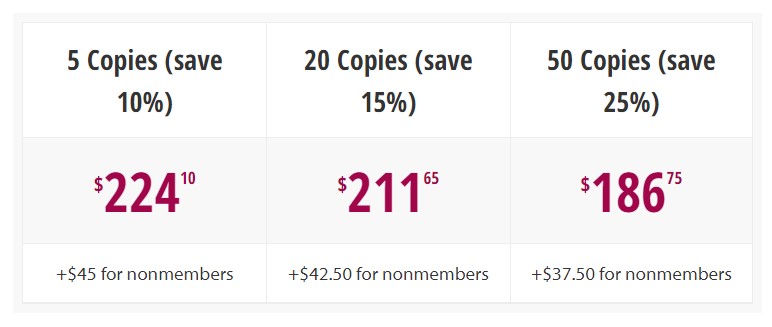
Bulk Rate Discounts Available. See More.
Internet Explorer and Microsoft Edge are not supported by the checkout process.
Please use Chrome, Firefox, or Safari. If you are unable to use these browsers, please contact us at 202-371-1420 or [email protected] and we will assist you.
FAQs
Review copies for professors who are adopting a book for a course are available for a reduced fee. Please contact [email protected] or 202-371-1420 for more information.
For bookstore bulk pricing, please email [email protected].
Yes. Orders of more than 5 books of a single title are eligible for discounts. Larger orders are eligible for larger discounts. Add copies to your cart or contact [email protected] (or call 202-371-1420) for more information!
Electronic books (e-books) are available for many titles. E-books are delivered to you through the VitalSource Bookshelf application, accessible on most mobile devices and computers. View supported devices. After receiving your order, an access code is sent to your email along with directions to create an account and redeem it. E-books are intended for individual use. If you would like a copy of the book that you plan to share with others, FDLI recommends purchasing a companion print book.
E-book orders are fulfilled as quickly as possible during business hours (usually within one business day) and are not available for immediate download upon checkout.
The e-book platform provides a number of benefits, including in-book annotation, an option for offline reading, and cross-platform syncing. Additionally, the e-book may be accessed through two mobile devices and two desktop computers at one time. If you are interested in learning more about the features, you can find a full list on VitalSource’s website.
Physical copies of books purchased through our website are printed and shipped directly to customers. Printing and shipping times may fluctuate, but the process usually takes 3–10 business days to print in addition to standard wait times for UPS Ground shipping (2–4 days). If you would like to expedite the shipping of your book, we can do so for an additional fee. Please contact us at [email protected] or 202-371-1420 prior to your order.
Please contact [email protected] for more information.