The DEA Quota System
by Lee H. Rosebush and Marc N. Wagner
While lately there has been a lot of focus on the prescribing and dispensing of controlled substances, it is important to remember there are many other areas of the distribution network that the Drug Enforcement Administration (DEA) regulates. On July 16, 2018, the DEA published a final rule pertaining to the quota system, which takes effect on August 15, 2018.1 Congress, in the Controlled Substances Act (CSA), created a “closed system” of distribution for controlled substances whereby each handler along the supply chain is registered with DEA and each transaction is accounted for. Along with this strict oversight, Congress also vested broad authority with the Attorney General to regulate the use of controlled substances in the medical, scientific research, and pharmaceutical manufacturing areas. For example, Section 306 of the Controlled Substances Act (21 U.S.C. § 826) requires the Attorney General to establish aggregate production quotas (APQs) for each basic class of controlled substance listed in Schedules I and II and for the List I Chemicals ephedrine, pseudoephedrine, and phenylpropanolamine. The Attorney General delegated this function to the Administrator of the DEA under 28 C.F.R. § 0.100.2 For substances listed in Schedules I and II and List I Chemicals, “DEA’s statutory responsibility is twofold: to prevent diversion and abuse of these drugs while ensuring an adequate and uninterrupted supply.”3
The DEA quota system affects patients, medical practitioners, scientists, and industry alike. Understanding how the DEA quota system works will help those affected by it, especially bulk active pharmaceutical ingredients (API) manufacturers, drug manufacturers, packagers, repackagers, labelers, relabelers, and 503B Outsourcing Facilities. Likewise, with increased attention on the opioid crisis, the DEA quota system is one tool that could be used to help curb the opioid crisis.
Controlled Substances Listed in Schedule I and II
For controlled substances listed in Schedule I and II, DEA sets three quotas: the Aggregate Production Quota (APQ),4 Manufacturing Quotas (MQ),5 and
Procurement Quotas (PQ).6
Unless otherwise stated, all quotas that will be discussed are for each basic class of controlled substance listed in Schedules I and II. Pursuant to 21 C.F.R. § 1300.01(b), basic class essentially encompasses all the chemical forms of the drug. For example, morphine is the basic class which encompasses all salt forms of morphine, including but not limited to morphine hydrobromide, morphine hydrochloride, and morphine sulfate.7
Aggregate Production Quota (APQ)
In general, DEA typically establishes one umbrella-like quota known as the APQ, which should be representative of the national need for a particular substance.8 As a federal agency, DEA follows the procedures set forth in the Administrative Procedure Act—namely informal notice and comment rulemaking procedures. To start the calculation process, DEA determines the “total quantity of each basic class of controlled substance listed in Schedule I or II necessary to be manufactured during the following calendar year.”9 The APQ should reflect medical, scientific, research, industrial, export, and reserve needs of the United States.10 To calculate each APQ, the DEA takes into account various factors such as the sum of manufacturing quotas, an FDA estimate, and prescription audit data.11 Notice of the annual APQ for each basic class of controlled substance listed in Schedules I and II is published in the Federal Register, and a copy is mailed to each person registered as a bulk manufacturer of the basic class.12 Interested parties may comment or object on the APQ. 13 DEA may, but is not required to, hold a hearing to discuss comments and objections to the APQ.14 In the event that a hearing is held, the rule making procedures set forth in the Administrative Procedure Act govern the hearing.15
After any such hearing and consideration of comments and objections, DEA publishes a final order determining the APQ for each basic class of controlled substance. This final order includes findings of fact and conclusions of law upon which the order is based and also specifies the date on which the order takes effect.16
Indeed, the fixed APQ published as a final order can be adjusted throughout the year. 17 DEA may increase or decrease the APQ for a basic class of controlled substances listed in Schedule I or II based on various factors, including but not limited to changes in the national rate of net disposal of the class, changes in demand for that class, and changes in the currently accepted medical use of the class or substances manufactured from the class.18 If DEA decides to increase or decrease the APQ, the same notice and comment procedure takes place as when the APQ is set for the year—interested parties may comment or object but DEA does not have to hold a hearing.19 Agencies need not respond to every comment but agencies must respond in a reasoned manner to significant comments received.20 Adjustments to the APQ are published in the Federal Register and mailed to each person registered as a bulk manufacturer.21
Manufacturing Quota (MQ)
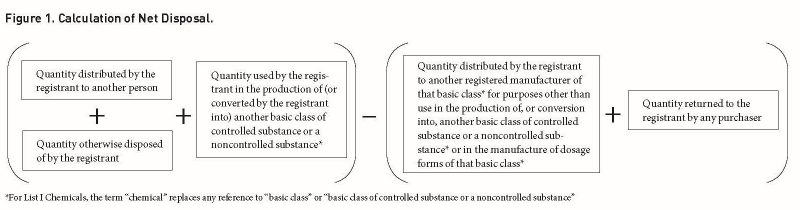
The MQ is the maximum amount that may be manufactured in a given year.22 The MQ is individual to each DEA registered manufacturer of a basic class of controlled substance listed in Schedule I or II.23 Manufacturers must apply for a MQ using DEA Form 189.24 DEA allocates MQs to current manufacturers based on the estimated net disposal of the manufacturer for the year.25 Net
disposal is calculated pursuant to
21 C.F.R. § 1300.01(b), displayed by
Figure 1 on the next page.
For new manufacturers of a basic class, the MQ is based on the reasonably estimated net disposal of that applicant for the next calendar year.26 Additionally, when assigning MQs to registrants, the DEA has broad discretion to adjust the allotment based on any factor the DEA deems relevant to fixing the MQ of the applicant.27 If a registrant disagrees with the MQ set by DEA or wants an increase, the registrant may file an application on DEA Form 189 for an increase in quota.28 MQs factor in that manufacturers are allotted an inventory allowance. Manufacturers may maintain an inventory equal to 50 percent of the average between the estimated net disposal for the current year and the last year, or for new manufacturers, 50 percent of the reasonably estimated net disposal.29 Manufacturers may not exceed an inventory of 65 percent of the estimated net disposal.30 In the event that the inventory allowance is exceeded, the MQ is suspended until inventory is less than 60 percent of net disposals.31 Similar to how the APQ may be adjusted, either increased or decreased, DEA may also increase or decrease the MQ for a registrant at any time.32 In the event that DEA holds a hearing to address comments regarding MQs, the adjudication procedures set forth in the Administrative Procedure Act govern.33 The fact that DEA may decrease the MQ at any time spurs legal questions. If DEA grants a MQ but later reduces the MQ, is this considered a regulatory taking? Other than requesting a hearing, holders of MQs have little recourse short of an arbitrary and capricious argument in court. Arguments abound for firms that have an MQ reduced. Additionally, “the judiciary has a responsibility under the APA to set aside agency actions that are ‘arbitrary, capricious, an abuse of discretion, or otherwise not in accordance with law.’”34 Courts will not set aside agency decisions when the agency “examined the relevant data and articulated a satisfactory explanation for its action including a rational connection between the facts found and the choice made.”35
Procurement Quotas (PQ)
The final type of quota, PQ limits the quantity an authorized person may procure and use of each basic class of substance for the purpose of manufacturing into dosage forms or other substances.36 Therefore, depending on the activity, some registrants may be required to have both a MQ and PQ.37 Like MQs, registrants must apply for a PQ. A separate application on DEA Form 250 must be submitted for a PQ for each basic class.38 Likewise, PQs apply to any person manufacturing dosage forms, including dosage form manufacturers, packagers, repackagers, labelers, and relabelers.
List I Chemicals
In addition to the quotas set for each basic class of controlled substance listed in Schedule I and II, DEA establishes four separate quotas for certain List I Chemicals: Assessment of Annual Needs (AAN),39 Manufacturing Quotas (MQ),40 Procurement Quotas (PQ),41 and Import Quotas (IQ).42 For purposes of this article, “List I Chemicals” will refer to only ephedrine, pseudoephedrine, and phenylpropanolamine, because these three chemicals are the only List I Chemicals subject to the quota system.
Assessment of Annual Needs (AAN)
The AAN for products containing ephedrine, pseudoephedrine, and phenylpropanolamine is nearly identical to fixing the APQ for controlled substances listed in Schedules I and II. DEA determines the total quantity of List I Chemicals necessary to be manufactured and imported during the following calendar year to provide for medical, scientific, research, industrial, export, and reserve needs of the United States.43 Again, DEA has broad discretion in assessing the annual needs.44 General notice of an AAN for List I Chemicals is published in the Federal Register, and a copy is mailed to each person registered to manufacture or import the chemical.45 Interested parties are allowed to comment or object on the AAN.46 DEA may hold a hearing to discuss comments and objections to the AAN; after any such hearing, DEA publishes a final order of the AAN specifying the effective date, including findings of fact and conclusions of law upon which the order is based.47 As seen with APQs, DEA may adjust the AAN at any time during the year.48 The factors DEA must consider in determining an adjustment to the AAN are listed in the Code of Federal Regulations.49
Manufacturing Quotas (MQ)
DEA follows the same process to set the MQ for individual manufacturers of ephedrine, pseudoephedrine, and phenylpropanolamine as for individual manufacturers of controlled substances listed in Schedules I and II. For a review of the process for fixing MQs, see Manufacturing Quotas (MQs) as discussed above in regards to controlled substances listed in Schedule I and II.50 Manufacturers must apply for a MQ using DEA Form 189.51 The MQ is the maximum amount that may be manufactured in a given year. DEA allocates MQs to current manufacturers based on the estimated net disposal of the manufacturer for the year.52 For List I Chemicals, net disposal is calculated pursuant to 21 C.F.R. § 1315.02(b), illustrated by Figure 1 above.
Just like MQs for controlled substances listed in Schedules I and II, DEA has broad discretion to adjust the allotment based on any factor DEA deems relevant to fixing the MQ of the applicant.53 Some of the factors include the applicant’s production cycle and current inventory, stability problems, and potential disruptions to production.54 The same set of rules apply for inventory allowance of List I Chemicals as apply for controlled substances listed in Schedules I and II as well.55 Furthermore, DEA may either increase or decrease the MQ for a registrant at any time.56
Procurement Quotas (PQ)
The PQs limit the quantity an authorized person may procure and use of each chemical for the purpose of manufacturing into dosage forms or other substances.57 For an explanation of PQs, see Procurement Quotas (PQs) as discussed above in regards to controlled substances listed in Schedules I and II.58 It is noteworthy that PQs apply to dosage form manufacturers, packagers, repackagers, labelers, and relabelers.
Import Quotas (IQ)
An IQ permits a registered importer to import List I Chemicals for either distribution to a registered manufacturer that has a PQ or for distribution consistent with legitimate medical and scientific uses.59 Importers apply for an IQ for each chemical on DEA Form 488.60 Like the other quotas, a separate application must be made for each chemical to be imported.61 Interestingly, the regulations do not contain any provision authorizing DEA to decrease an IQ. An IQ can be increased at any time at the request by the person whom an IQ was issued to if the DEA determines that an increase is necessary for medical, scientific, or other legitimate purposes.62 Additionally, if DEA does not respond to a request for an increase in IQ within 60 days of receiving the application, the application is deemed to be approved until DEA notifies the applicant that the approval is terminated.63
What Does the Final Rule Add to the Quota System?
Arguably, the Final Rule issued on July 16, 2018 is an effort to recommit DEA to control diversion of controlled substances. Despite the 1,561 written and electronic comments DEA received in merely a 15-day comment period, the Agency finalized the proposed rule from April 19, 2018 without changes.64 The final rule contains four significant additions to the quota system. First, the final rule mandates that when DEA is fixing the APQ, the agency must consider the extent of any diversion of controlled substance in addition to relevant information from HHS, FDA, CDC, CMS, and the states.65 Second, when issuing PQs and MQs, the final rule allows DEA to request “additional information from an applicant which . . . may be helpful in detecting or preventing diversion, including customer identities and amounts of the controlled substance sold to each customer.”66 Third, when fixing or adjusting an MQ, DEA must consider “the extent of any diversion of the controlled substance.”67 Fourth and finally, the copies of general notice for setting the APQ and the final order will now be transmitted to each state attorney general, providing the states an opportunity to comment or object.68
Aside from involving the states in the quota process, the final rule does not add anything substantive to the DEA quota system. Arguably, DEA should have been considering the extent of diversion and all relevant information from other agencies when fixing and adjusting quotas prior to the final rule. Additionally, DEA already collects information regarding transactions with controlled substances listed in Schedules I and II through the Automation of Reports and Consolidated Orders System (ARCOS) so no new information will be collected by the DEA even if the agency requests customer identities.69
Quota Considerations
The DEA quota system is one control ensuring the United States has enough controlled substances listed in Schedules I and II and List I Chemicals to adequately supply medical, scientific, research, and industrial needs. Similarly, the DEA quota system is one control to limit the amount of drug introduced into the supply chain—a preventative measure against diversion. The recent final rule on Controlled Substances Quotas makes clear DEA’s intent to use the quota system to prevent diversion. It is essential to obtain regulatory counsel when dealing with the DEA quota system. Questions remain unanswered. For instance, PQs apply to dosage form manufacturers, packagers, repackagers, labelers, and relabelers. If the same dosage form is manufactured, subsequently repackaged, and later relabeled, is that quantity double or triple counted when determining the APQ? How do 503B registered Outsourcing Facilities affect the APQ? If an MQ is decreased mid-year, does this implicate a regulatory taking? Guidance or policy statements from the DEA to industry are welcome in the obscure world of DEA quotas.
- Controlled Substances Quotas, 83 Fed. Reg. 32,784 (July 16, 2018) (to be codified at 21 C.F.R. pt. 1303).
- 21 C.F.R. § 1300.01(b) (2018).
- U.S. DEP’T OF JUSTICE, DRUG ENFORCEMENT ADMINISTRATION, PRACTITIONER’S MANUAL, AN INFORMATIONAL OUTLINE OF THE CONTROLLED SUBSTANCES ACT, 4 (2006)
- 1303.11(a).
- 1303.21(a).
- 1303.12(a).
- 1300.01(b)(1).
- See Minh T. Dang, Quotas, DRUG ENFORCEMENT ADMINISTRATION, slide 19, https://www.deadiversion.usdoj.gov/mtgs/man_imp_exp/conf_2013/dang_1.pdf.
- 1303.11(a).
- Id.
- Dang, supra note 8 at 25. See also § 1303.11(b).
- 1303.11(c).
- Id.
- Id.
- 1303.31(a).
- 1303.11(c).
- 1303.13(a).
- 1303.13(a). For a list of the factors the DEA considers in adjusting APQs, see § 1303.13(b).
- 1303.13(c).
- See United States v. Nova Scotia Food Prods Corp., 568 F.2d 240, 252−53 (2d Cir. 1977).
- 1303.13(c).
- 1303.21(a).
- Id.
- 1303.22.
- 1303.23(a).
- 1303.23(b).
- 1303.23(a)(2), (b)(2). When fixing the MQ for an individual manufacturer, DEA may consider “any other factors which the Administrator deems relevant to the fixing of the individual manufacturing quota of the applicant, including the trend of (and recent changes in) his and the national rates of net disposal, his production cycle and current inventory position, the economic and physical availability of raw materials for use in manufacturing and for inventory purposes, yield and stability problems, potential disruptions to production (including possible labor strikes), and recent unforeseen emergencies such as floods and fires.” § 1303.23(a)(2).
- 1303.25(a).
- 1303.24(a).
- 1303.24(b).
- Id.
- § 1303.25, 1303.26.
- 1303.31(b). Adjudication procedures apply in hearings for MQs because MQs are individualized whereas rulemaking procedures apply in hearings regarding the APQ because the APQ has general applicability and future effects.
- MD Pharm., Inc. v. DEA, 133 F.3d 8, 16 (D.C. Cir. 1998) (internal quotation marks and citations omitted) (holding that DEA gave an adequate explanation for its decision to register Mallinckrodt even though MD Pharm., Inc. objected to Mallinckrodt’s registration as a bulk manufacturer of methylphenidate, a controlled substance listed in Schedule II).
- Id. (citing Motor Vehicle Mfrs. Ass’n v. State Farm Mut. Auto. Ins. Co., 463 U.S. 29, 43 (1983)).
- 1303.12(a).
- Dang, supra note 7 at 41.
- 1303.12(b).
- 1315.11(a).
- 1315.21.
- 1315.30(a).
- Id.
- 1315.11(a).
- See § 1315.11(b) (listing the factors the DEA must consider when determining the AAN).
- 1315.11(c).
- 1315.11(d).
- 1315.11(e) to (f).
- 1315.13(a).
- 1315.13(b).
- See also §§ 1315.23, 1315.25, 1315.26.
- 1315.22.
- 1315.23(a).
- 1315.23(b).
- Id.
- 1315.24.
- 1315.25, 1315.26.
- 1315.30(b).
- The same rules apply for PQs of List I Chemicals (Ephedrine, Pseudoephedrine, and Phenylpropanolamine) as controlled substances listed in Schedules I and II. The applicable regulations differ. See 21 C.F.R. §§ 1315.30 to 1315.32 and §§ 1315.34 to 1315.36.
- 1315.30(c).
- 1315.34(a).
- Id.
- 1315.36(b).
- 1315.36(c).
- Controlled Substances Quotas, 83 Fed. Reg. 32,784, 32,785 (July 16, 2018) (to be codified at 21 C.F.R. pt. 1303).
- Id. at 32,784.
- Id. at 32,790.
- Id.
- Id. at 32,789.
- See id. at 32,788.
Programs
Update Magazine
August/September 2018