New Bioengineered (aka GM) Food Disclosure Law: Useful Information or Consumer Confusion?
By Gregory Jaffe and Jennifer Kuzma
Farmers began growing genetically modified (GM) crops in 1996, and today, U.S. farmers grow GM varieties of ten crops, including the vast majority of U.S. acreage of corn, soybean, upland cotton, sugar beets, and canola. However, their introduction has not been without public controversy, including calls to label food products made from those crops. In 2016, Congress passed, and President Obama signed, the National Bioengineered Food Disclosure Law (NBFDL), establishing an obligation for food manufacturers to disclose to consumers whether their food products are bioengineered or contain bioengineered ingredients. The U.S. Department of Agriculture (USDA) finalized regulations for implementing that law in late 2018, requiring food manufacturers to comply by January 1, 2022.
In this article, we explore whether the law and associated regulations provide consumers who want to know whether foods are bioengineered with adequate information to make informed decisions about bioengineering as part of their food choices. We first describe the events that led up the passage of the NBFDL and then briefly explain how the law will operate. Then, we analyze how the law will be implemented and whether it will inform or confuse consumers. Finally, we provide recommendations for regulatory revisions and consumer education that are necessary to make the law useful to consumers.
Background
Until 2016, there was no mandatory requirement to label or disclose whether foods came from GM crops. The U.S. Food and Drug Administration (FDA), the agency tasked with regulating food safety and nutrition labeling for most of the U.S. food supply, has stated that “bioengineered foods do not differ in any meaningful or uniform way or present any different or greater safety concern than food developed from traditional breeding.” They also concluded that “the method of development of a new plant variety is generally not material information . . . and would not usually be required to be disclosed in the labeling for the food.”[i] However, they did develop a guidance for industry, which they finalized in 2015 and revised in 2019, that allowed manufacturers on a voluntary basis to label foods with information about whether the food was or was not derived from genetically engineered plants.[ii]
To date, many manufacturers have voluntarily labeled foods as “non-GMO” using various standards established by NGOs and industry. However, very few, if any, food manufacturers voluntarily labeled foods as affirmatively containing GM ingredients. Separately, the National Organic Program at the USDA excludes foods made with GM ingredients from being labeled as “organic” (7 U.S.C. 6524). Consumers seeking to avoid GM products have been able to do so by buying organic, although often those foods are more expensive than non-organic foods. They can also purchase foods with a “non-GMO” designation, or foods made solely from crops and animals that are not GM. However, for many consumers wishing to avoid GM products, the marketplace is confusing.
Congress Passes the National Bioengineered Food Disclosure Law
In 2016, Congress passed, and President Obama signed, the NBFDL, establishing an obligation for food manufacturers to disclose to consumers whether their food products are bioengineered or contain bioengineered ingredients.[iii] Passage of the NBFDL was largely prompted by (1) polls and other data supporting mandatory labeling of GM foods; and (2) legislative action at the state level that could have created a patchwork of different requirements. Studies show that, overall, consumers desire GM food labels and prioritize them over other types of labels.[iv] Consumers are willing to pay a premium for foods labeled as non-GM over those labeled as GM under different labeling conditions proposed by the NBFDL,[v] although there is heterogeneity among types of consumers in their GM food choices and labeling preferences.[vi]
Prior to the NBFDL, consumer and organic foods advocacy groups pushed for GM labeling laws, and many states had introduced bills and ballot initiatives to require mandatory GM labeling. In 2016 alone, 70 bills were introduced in 25 states to address the labeling of GE foods.[vii] Three states—Vermont, Maine, and Connecticut—enacted mandatory labeling laws.[viii] However, it was the Vermont law that pushed Congress to act as it went into effect on July 1, 2016, forcing food companies to begin labeling their food packages. The need to label all their food products because of the Vermont law (because food companies do not produce products for just one state) and the possibility of a confusing patchwork of GM labeling requirements if other states enacted laws with different obligations were likely to be burdensome to food manufacturers, producers, and suppliers.[ix]
The NBFDL preempted all state laws, rendering the Vermont law moot. The federal government would become the place for mandatory bioengineered disclosure instead of state governments. USDA’s Agricultural Marketing Services, not FDA, was designated as the agency to oversee implementing bioengineered disclosure.[x]
The Mechanics of Disclosure under the NBFDL and USDA’s Regulations
Who Discloses and How
The NBFDL[xi] and USDA’s regulations[xii] put the burden of disclosing GM products and ingredients on food manufacturers, who can make the disclosure in four different ways: (1) a textual description on the package that the food is “bioengineered” or “contains bioengineered ingredients”; (2) a USDA-designed symbol on their package indicating “bioengineered” (Figure 1); (3) an electronic or digital link on the package, which when scanned by the consumer, goes to a website disclosure; or (4) a telephone number on the package that consumers can text to receive the disclosure.
When the law was being developed, many food manufacturers pushed for the electronic or digital-link option. That option requires consumers to take an active step to access the information at the point of sale. An electronic QR code would be on the package, which consumers scan to get the disclosure information. Since the bioengineered content information is provided to the consumer electronically, the law is characterized as requiring “disclosure” rather than “labeling.”
What Terms Can Be Used.
The NBFDL uses “bioengineered” but provides USDA with the discretion to allow other “similar” terms.[xiii] In the regulations, USDA determined that “bioengineering and bioengineered food accurately reflected the disclosure and the products and potential technology at issue,” and that using additional terms might cause marketplace confusion.[xiv] Thus, manufacturers may use only the term “bioengineered” and are prohibited from using “genetically engineered,” “genetically modified,” or “GMO”—terms more commonly understood by consumers.
The manufacturer either states the food product is “bioengineered” (if all ingredients are bioengineered) or “contains bioengineered ingredients.” The regulations prohibit the manufacturer from identifying specific ingredients from bioengineered organisms. The same terms and language must be used for the electronic and text disclosure options.[xv]
Which Foods Do and Do Not Require a Disclosure
The NBFDL defines “bioengineered” as any food “(a) that contains genetic material that has been modified through in vitro recombinant DNA techniques; and (b) for which the modification could not otherwise be obtained through conventional breeding or not found in nature.”[xvi] USDA’s regulations interpreted this definition to only cover foods with detectable levels of altered genetic material (e.g., a piece of GM sweet corn) or food products with ingredients containing detectable levels of altered genetic material (e.g., frozen mixed vegetables with GM sweet corn).[xvii] USDA decided that refined products originating from bioengineered crops but without engineered DNA are not “bioengineered.” Foods containing highly refined ingredients such as soybean oil from GM soybeans or sugar from GM sugar beets will not require disclosure. That decision is consistent with the GM labeling requirements in some countries (e.g., Japan and Australia) but not others (e.g., the European Union). USDA published a list of bioengineered crops and animals that need disclosure and will update that list periodically.[xviii]
The NBFDL and regulations do not require disclosure for certain bioengineered foods including: (1) restaurant food; (2) foods produced by “very small manufacturers,” defined as having receipts less than $2,500,000; (3) food made from animals fed bioengineered organisms; and (4) foods where one or more ingredients have BE content that is inadvertent or technically unavoidable if it is not more than 5% of any ingredient.[xix]
The disclosure requirement applies to all food regulated by FDA but does not apply to certain foods containing pork, beef, sheep, goat, catfish, chicken, turkey, domesticated birds, and egg products, which are regulated by USDA.[xx] Bioengineered animals, except for fish, seafood, and game animals, and foods that contain ingredients from those animals are exempt. The law and regulations require disclosure for foods containing non-bioengineered meat, poultry, or eggs which contain a bioengineered ingredient if either: (1) the first ingredient is something other than meat, poultry, or egg; or (2) the first ingredient is water, stock, or broth and the second ingredient is something other than meat, poultry, or eggs.[xxi]
What Can Be Voluntarily Disclosed
While food manufacturers wanted to voluntarily disclosure additional details about bioengineered foods, USDA’s final rule limits voluntary disclosures. The regulations only allow manufacturers to voluntarily disclose in two situations.[xxii]
First, a manufacturer may disclose that a refined food or ingredient in that food originated from a bioengineered food, such as disclosing that corn syrup in a carbonated beverage originated from bioengineered corn. The refined product disclosure can state “derived from bioengineering” or “ingredient(s) derived from a bioengineered source,” and the word “ingredient” can be replaced with the specific crop or food ingredient (contrary to the mandatory “bioengineered” disclosure, which does not permit identifying specific bioengineered ingredients).[xxiii] The voluntary disclosure also can use a USDA-designed “derived from bioengineering” symbol (Figure 1).
The second voluntary disclosure allowed is for very small food manufacturers, who are exempt from mandatory disclosure but can voluntarily disclose that their foods are bioengineered or contain bioengineered-derived ingredients.[xxiv]
Analysis
When the bioengineered foods disclosure requirement is implemented in 2022, will it provide information useful to consumers or will it lead to more confusion? While the NBFDL establishes mandatory disclosure for bioengineered food content, the information disclosed may not be useful for several reasons.
The Disclosure Itself is Confusing. The NBFDL switches from the common term “GM,” which is more familiar to consumers, to “bioengineered” (BE). The intent of this change may have been to avoid the contentious history of “GM foods” by renaming them, but it could instead undermine consumer trust if consumers see it as a tactic to mask terms they commonly understand.[xxv]
Also, the regulations do not allow for manufacturers to identify the specific bioengineered ingredients in their product, which could result in consumers making incorrect assumptions about what is bioengineered in the food supply. For example, a frozen vegetable pizza with some GM green squash would be disclosed as “contains a bioengineered ingredient.” However, the consumer is likely to incorrectly assume that one of the major ingredients in pizza—the wheat, the tomato sauce, or the cheese—is bioengineered, not a minor ingredient such as squash. Disclosure by ingredient, which is required in the European Union, would provide useful information to consumers instead of consumers potentially misinterpreting the scope of the product’s bioengineering.
The Electronic Option May Not Be Accessible. By allowing manufacturers to choose their disclosure option, the regulations may make it difficult for some consumers to access the information, especially at the point of sale. Some consumers may not know how to scan electronic codes or realize that they can get the disclosure from a telephone text. A Deloitte study commissioned by USDA found: “Of 40 in-depth conversations with consumers, all 40 either did not recognize the on-package digital link or did not associate it with food information. Retailers were also unfamiliar with digital links and thus were unable to assist consumers.”[xxvi] Furthermore, in rural areas, access to broadband internet is an issue. Recently, a lawsuit was initiated challenging USDA’s regulations, claiming that some disclosure options are discriminatory since not everyone has a smartphone or internet access.[xxvii] It is currently not clear whether manufacturers will choose the digital disclosure option. Recently, one major food company, Ahold Delhaize USA, announced that its private label products will have “clear on-package Bioengineered Food labeling.”[xxviii]
While an electronic disclosure may be difficult for some consumers to access, it does have some advantages. Stating that a food has bioengineered content does not really provide sufficient information for many consumers, who might want to know not only which ingredients came from genetic engineering, but why genetic engineering was used. By providing the information on a website, the food manufacturer can provide additional information other than the required few word disclosure and link to additional resources, resulting in consumer education on the topic.
The Exemptions are Confusing. Excluding highly refined ingredients without “modified DNA” will significantly limit the number of food products requiring disclosure. It is unclear whether manufacturers will voluntarily disclose highly refined ingredients derived from bioengineered crops. During the rule-making process, some manufacturers advocated for disclosing highly refined ingredients because that’s what their consumers want, they wanted to be more transparent, or they wanted uniform disclosures.[xxix] Manufacturers provided evidence to USDA that consumers expected these products to have a disclosure and that they wanted to meet consumer expectations.[xxx] If there are two identical products (e.g., corn oil), but only one makes a voluntary disclosure, consumers might make their choice based on a distinction that does not exist.
Additionally, the exemption for certain products that have ingredients regulated by USDA will make it difficult for consumers to be confident that a food without a disclosure is not bioengineered. For products like soups or pizzas with meat ingredients, the relative quantity of meat in the product will determine if it requires disclosure. As an illustration, Figure 2 has the label of two Progresso chicken noodle soups. Disclosure is not required if chicken is the first or second ingredient (after broth or water), but it is required when it is the third ingredient. Determining whether a product falls within an exemption will require consumers to understand the exemptions and then closely read the ingredient list.
USDA Did Not Address Absence Claims. The law and regulations do not address the issue of absence claims, which are claims that a product does not contain bioengineered ingredients. The law states that any certified “organic” food can make an absence claim as the National Organic Standards exclude GM ingredients from being certified as organic (7 U.S.C. 6524). Also, the law states that if a food does not require disclosure as “bioengineered,” it does not mean it can claim to be “not bioengineered” or “non-GMO.”[xxxi] USDA did not include in the regulations any provisions specifying when a food can be labeled “not bioengineered” or “non-GMO.”
For many years, private labeling bodies and individual companies have set their own standards for when a food is “non-GMO.” Non-GM foods sales increased from $12.9 billion in 2012 to $21.2 billion in 2016, and 46% of U.S. consumers report actively avoiding GM foods.[xxxii] Those claims receive no oversight by USDA. They may be covered by FDA’s guidance on voluntary labeling for foods from GM plants.[xxxiii] However, that guidance only applies to FDA-regulated food products, not products with meat or poultry ingredients, and FDA has not enforced compliance for absence claims. Currently, non-GM claims can be misleading to consumers, as some non-GMO claims are made on products where there are no GM varieties (e.g., 100% orange juice when no GM oranges exist).[xxxiv] USDA could have cleared up this confusion in its regulations but chose not to do so.
Conclusion: A Better Path Forward
The impacts of the NBFDL remain to be seen, but it is apparent that when the disclosures arrive, it is likely to be confusing to consumers for all the reasons stated above. The disclosure will enter a marketplace with an already-confusing landscape of GM-related information (including organic and non-GMO claims) (Figure 1).
To remedy the confusion, we propose four interventions. First, we believe there should be education and information dissemination campaigns by the federal government (primarily FDA and USDA), food manufacturers, and retailers. Implementation will require resources to FDA and USDA and coordination between federal agencies and industry. Those campaigns should explain the NFBDL so consumers can access and understand the information being provided. The campaign should introduce consumers to the term “bioengineered,” explaining that this is the same as “GMO,” or “genetically engineered.” The campaign also should provide information about accessing the disclosure, as well as alert consumers to the exemptions and types of voluntary disclosures. Finally, an education campaign should provide information about the lack of food safety or nutritional differences between bioengineered and conventional foods and information explaining that risks or benefits vary by product. FDA was tasked by Congress several years ago to develop an educational initiative to better understand GM foods. The result of this initiative has been the publication of fact-based information on their website, which is a useful resource.[xxxv] However, FDA’s information does not explain the disclosure requirements in detail, nor does FDA have the resources to reach the average consumer with that information.
Secondly, given the consumer confusion that the regulations are likely to cause, USDA should reconsider whether to allow the substitution of similar terms to bioengineering, such as “genetic engineering” or GM. As described above, the USDA rules currently do not allow for these substitutions.
Third, USDA should reconsider disclosure of highly refined ingredients in recognition that consumers would be best served by consistent disclosure of products “derived from bioengineering.” USDA should also allow manufacturers to specify the bioengineered ingredients. This would enable consumers to associate bioengineering with specific crops and ingredients, educating them about where the technology is used in agriculture and food and providing detailed information about which bioengineered ingredients are in a food.
Finally, USDA should consider regulating absence claims. A national standard would provide uniformity ensuring consumers who want to purchase non-GMO foods are getting what they paid for.
In conclusion, now is the time for the government and the food industry to address the potential consumer confusion before the disclosures in 2022 result in consumer frustration and mistrust. If the goal of greater information to consumers is to be realized, USDA should rethink its regulations and consumers should be educated about the disclosures being provided.
Figures
Figure 1: Different Information That Will be Available to Consumers Starting in 2022.
The text in red and italics in each column represents the categories to which labels in the corresponding column would apply. Black text in each column represents the text that would constitute the food label.
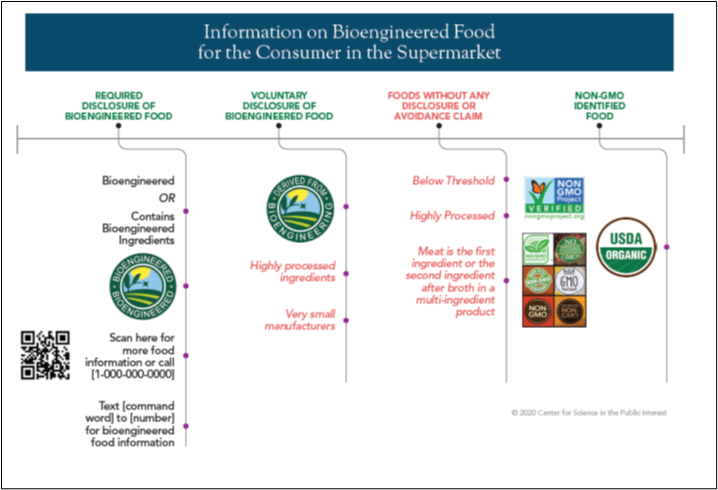
Figure 2: Example of Consumer Confusion Through Two Cans of Soup
The figure shows how the NBFDL applies to products that contain meat as a major ingredient. The first soup can for Progresso Chicken Noodle Soup (A and B) has broth as a first ingredient and chicken as the second ingredient; it is not covered by the NBFDL and will not disclose if any ingredients are bioengineered. The second soup can for Progresso Roasted Chicken Noodle Soup (C and D) has broth as the first ingredient, carrots as the second ingredient, and chicken as the THIRD ingredient; this soup is covered by NBFDL and will require disclosure if it has bioengineered ingredients.

[i] U.S. Food & Drug Admin., Voluntary Labeling Indicating Whether Foods Have or Have Not Been Derived from Genetically Engineered Plants: Guidance for Industry (March 2019), https://www.fda.gov/media/120958/download (last visited Sept. 4, 2020).
[ii] Id.
[iii] National Bioengineered Food Disclosure Standard Act, 7 U.S.C. § 1639 (2016).
[iv] Jennifer Kuzma, Society and Policy Makers’ Responsibilities, in Consumer Perception of Product Risks and Benefits (Gerard Emilien, Rolf Weitkunat & Frank Lüdicke, eds. 2017).
[v] Brandon R. McFadden & Jayson L. Lusk, Effects of the National Bioengineered Food Disclosure Standard: Willingness to Pay for Labels that Communicate the Presence or Absence of Genetic Modification, 40 Applied Econ. Persp. & Pol’y 259 (2018); Brandon R. McFadden & Trey Malone, How Will Mandatory Labeling of Genetically Modified Food Nudge Consumer Decision-Making?, 77 J. Behav. & Experimental Econ. 186 (2018).
[vi] McFadden & Malone, supra note 5; Gina Waterfield, Scott Kaplan & David Zilberman, Willingness to Pay versus Willingness to Vote: Consumer and Voter Avoidance of Genetically Modified Foods, 102 Am. J. Agric. Econ. 505 (2020); Chengyan Yue, Shuoli Zhao & Jennifer Kuzma, Heterogeneous Consumer Preferences for Nanotechnology and Genetic-Modification Technology in Food Products, 66 J. Agric. Econ. 308 (2015).
[vii] Doug Farquhar, State Legislation Addressing Genetically-Modified Organisms: GMO Labeling Summary, Nat’l Conference of State Legislatures (2016), available at www.ncsl.org/research/agriculture-and-rural-development/state-legislation-addressing-genetically-modifiedorganisms-report.aspx.
[viii] Id.
[ix] Brandon R. McFadden, The Unknowns and Possible Implications of Mandatory Labeling, 35 Trends in Biotechnology 1 (2017).
[x] National Bioengineered Food Disclosure Standard Act, 7 U.S.C. § 1639 (2016).
[xi] Id.
[xii] National Bioengineered Food Disclosure Standard, 83 Fed. Reg. 65814 (Dec. 21, 2018).
[xiii] 7 U.S.C. §1639.
[xiv] National Bioengineered Food Disclosure Standard, 83 Fed. Reg.
[xv] Id.
[xvi] 7 U.S.C. § 1639.
[xvii] National Bioengineered Food Disclosure Standard, 83 Fed. Reg.
[xviii] List of Bioengineered Foods, USDA (2020), https://www.ams.usda.gov/rules-regulations/be/bioengineered-foods-list (last accessed September 4, 2020).
[xix] 7 U.S.C. § 1639; National Bioengineered Food Disclosure Standard, 83 Fed. Reg.
[xx] 7 U.S.C. § 1639.
[xxi] Id.; National Bioengineered Food Disclosure Standard, 83 Fed. Reg.
[xxii] National Bioengineered Food Disclosure Standard, 83 Fed. Reg.
[xxiii] Id.
[xxiv] Id.
[xxv] Jennifer Kuzma, Regulating Gene-Edited Crops, 35 Issues in Sci. & Tech. 80 (2018).
[xxvi] Study of Electronic or Digital Link Disclosure: A Third-Party Evaluation of Challenges Impacting Access to Bioengineered Food Disclosure, Deloitte (July 2017), https://www.agri-pulse.com/ext/resources/pdfs/USDADeloitteStudyofElectronicorDigitalDisclosure20170801.pdf.
[xxvii] Press Release, Center for Food Safety (CFS), Lawsuit Challenges “Bioengineered” GMO Food Labeling (July 28, 2020), https://www.centerforfoodsafety.org/press-releases/6100/lawsuit-challenges-bioengineered-gmo-food-labeling, (last accessed September 3, 2020).
[xxviii] GMO Position, Ahold Delhaize, https://www.aholddelhaize.com/en/about-us/stakeholder-interests/genetically-modified-organisms/ (last accessed September 4, 2020).
[xxix] Grocery Manufacturers Association, Comment Letter on National Bioengineered Food Disclosure Standard; Proposed Rule (July 3, 2018), https://www.regulations.gov/document?D=AMS-TM-17-0050-12345 (last accessed September 4, 2020).
[xxx] Id.
[xxxi] National Bioengineered Food Disclosure Standard Act, 7 U.S.C. § 1639 (2016).
[xxxii] Organic and Natural 2018, Hartman Group (July 2018), http://store.hartman-group.com/content/organic-and-natural-2018-study-overview.pdf; Hadley Malcolm, Non-GMO Demand Growing Despite Report that Says GMOs are Safe, USA Today (May 18, 2016), https://www.usatoday.com/story/money/2016/05/18/gmo-report-not-likely-to-change-minds-over-gmo-concern/84501686/.
[xxxiii] U.S. Food & Drug Admin., supra note 1.
[xxxiv] Greg Jaffe, Biotech Blog—Shopping for Honesty: Sorting Out Non-GMO Claims, Ctr. for Sci. in the Pub. Interest (April 17, 2017), https://cspinet.org/news/biotech-blog%E2%80%94shopping-honesty-sorting-out-non-gmo-claims-20170417 (last accessed September 4, 2020).
[xxxv] FDA’s “Feed Your Mind” website can be found at https://www.fda.gov/food/consumers/agricultural-biotechnology (last accessed April 9, 2021).
Update Magazine
Summer 2021

 GREGORY JAFFE is a lawyer who directs the Biotechnology Project at the Center for Science in the Public Interest, a consumer organization located in Washington, DC. Greg is a recognized expert in the regulation of biotechnology products both in the United States and around the world.
GREGORY JAFFE is a lawyer who directs the Biotechnology Project at the Center for Science in the Public Interest, a consumer organization located in Washington, DC. Greg is a recognized expert in the regulation of biotechnology products both in the United States and around the world. JENNIFER KUZMA, PhD, is the Goodnight-NCGSK Foundation Distinguished Professor in the School of Public and International Affairs, and co-founder and co-director of the Genetic Engineering and Society (GES) Center at NC State University. Prior to her current position, Dr. Kuzma was an associate professor at the Humphrey School of Public Affairs, University of Minnesota (2003-2013); study director at the National Academies of Science, Engineering, and Medicine (NASEM); and an AAAS Risk Policy Fellow at the USDA. She has over 140 scholarly publications on emerging technologies, their societal and ethical implications, and governance systems and has been studying these areas for over twenty-five years. Kuzma has held several national and international leadership positions, including a member of the World Economic Forum Council on Technology, Values and Policy; the NASEM Committee on Preparing for Future Biotechnology, Society for Risk Analysis (SRA) Council Member and Secretary, and the FAO Expert Group on Food and Nanotechnology. In 2019 she was elected a lifetime Fellow of American Association for the Advancement of Science (AAAS) for her distinguished work in anticipatory governance of new technologies, and methods for oversight policy analysis. In 2014, she received the SRA Sigma Xi Distinguished Lecturer Award for her contributions to the field of risk analysis and in 2017-2018 she was awarded the Fulbright Canada Research Chair in Science Policy.
JENNIFER KUZMA, PhD, is the Goodnight-NCGSK Foundation Distinguished Professor in the School of Public and International Affairs, and co-founder and co-director of the Genetic Engineering and Society (GES) Center at NC State University. Prior to her current position, Dr. Kuzma was an associate professor at the Humphrey School of Public Affairs, University of Minnesota (2003-2013); study director at the National Academies of Science, Engineering, and Medicine (NASEM); and an AAAS Risk Policy Fellow at the USDA. She has over 140 scholarly publications on emerging technologies, their societal and ethical implications, and governance systems and has been studying these areas for over twenty-five years. Kuzma has held several national and international leadership positions, including a member of the World Economic Forum Council on Technology, Values and Policy; the NASEM Committee on Preparing for Future Biotechnology, Society for Risk Analysis (SRA) Council Member and Secretary, and the FAO Expert Group on Food and Nanotechnology. In 2019 she was elected a lifetime Fellow of American Association for the Advancement of Science (AAAS) for her distinguished work in anticipatory governance of new technologies, and methods for oversight policy analysis. In 2014, she received the SRA Sigma Xi Distinguished Lecturer Award for her contributions to the field of risk analysis and in 2017-2018 she was awarded the Fulbright Canada Research Chair in Science Policy.